Early Humoral Response Correlates with Disease Severity and Outcomes in COVID-19 Patients
- PMID: 33291713
- PMCID: PMC7761967
- DOI: 10.3390/v12121390
Early Humoral Response Correlates with Disease Severity and Outcomes in COVID-19 Patients
Abstract
The Coronavirus Disease 2019 (COVID-19), caused by SARS-CoV-2, continues to spread globally with significantly high morbidity and mortality rates. Antigen-specific responses are of unquestionable value for clinical management of COVID-19 patients. Here, we investigated the kinetics of IgM, IgG against the spike (S) and nucleoproteins (N) proteins and their neutralizing capabilities in hospitalized COVID-19 patients with different disease presentations (i.e., mild, moderate or severe), need for intensive care units (ICU) admission or outcomes (i.e., survival vs death). We show that SARS-CoV-2 specific IgG, IgM and neutralizing antibodies (nAbs) were readily detectable in almost all COVID-19 patients with various clinical presentations. Interestingly, significantly higher levels of nAbs as well as anti-S1 and -N IgG and IgM antibodies were found in patients with more severe symptoms, patients requiring admission to ICU or those with fatal outcomes. More importantly, early after symptoms onset, we found that the levels of anti-N antibodies correlated strongly with disease severity. Collectively, these findings provide new insights into the kinetics of antibody responses in COVID-19 patients with different disease severity.
Keywords: COVID19; SARS-CoV-2; anti-N antibodies; neutralizing antibodies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


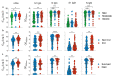
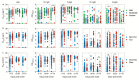
Similar articles
-
Longitudinal Profiling of Antibody Response in Patients With COVID-19 in a Tertiary Care Hospital in Beijing, China.Front Immunol. 2021 Mar 15;12:614436. doi: 10.3389/fimmu.2021.614436. eCollection 2021. Front Immunol. 2021. PMID: 33790892 Free PMC article.
-
A Highly Sensitive and Specific SARS-CoV-2 Spike- and Nucleoprotein-Based Fluorescent Multiplex Immunoassay (FMIA) to Measure IgG, IgA, and IgM Class Antibodies.Microbiol Spectr. 2021 Dec 22;9(3):e0113121. doi: 10.1128/Spectrum.01131-21. Epub 2021 Nov 17. Microbiol Spectr. 2021. PMID: 34787485 Free PMC article.
-
Quantitative SARS-CoV-2 Serology in Children With Multisystem Inflammatory Syndrome (MIS-C).Pediatrics. 2020 Dec;146(6):e2020018242. doi: 10.1542/peds.2020-018242. Epub 2020 Sep 2. Pediatrics. 2020. PMID: 32879033
-
Declining Levels of Neutralizing Antibodies Against SARS-CoV-2 in Convalescent COVID-19 Patients One Year Post Symptom Onset.Front Immunol. 2021 Jun 16;12:708523. doi: 10.3389/fimmu.2021.708523. eCollection 2021. Front Immunol. 2021. PMID: 34220870 Free PMC article.
-
Antibody Responses in COVID-19: A Review.Front Immunol. 2021 Apr 15;12:633184. doi: 10.3389/fimmu.2021.633184. eCollection 2021. Front Immunol. 2021. PMID: 33936045 Free PMC article. Review.
Cited by
-
Is Better Standardization of Therapeutic Antibody Quality in Emerging Diseases Epidemics Possible?Front Immunol. 2022 Feb 22;13:816159. doi: 10.3389/fimmu.2022.816159. eCollection 2022. Front Immunol. 2022. PMID: 35273599 Free PMC article.
-
SARS-CoV-2-Induced Immunosuppression: A Molecular Mimicry Syndrome.Glob Med Genet. 2022 Jul 14;9(3):191-199. doi: 10.1055/s-0042-1748170. eCollection 2022 Sep. Glob Med Genet. 2022. PMID: 35846107 Free PMC article.
-
Characterization of antibody response in asymptomatic and symptomatic SARS-CoV-2 infection.PLoS One. 2021 Jul 2;16(7):e0253977. doi: 10.1371/journal.pone.0253977. eCollection 2021. PLoS One. 2021. PMID: 34214116 Free PMC article.
-
Back to the Future: Immune Protection or Enhancement of Future Coronaviruses.Microorganisms. 2024 Mar 19;12(3):617. doi: 10.3390/microorganisms12030617. Microorganisms. 2024. PMID: 38543668 Free PMC article. Review.
-
Robust memory humoral immune response to SARS-CoV-2 in the tonsils of adults and children.Front Immunol. 2023 Dec 11;14:1291534. doi: 10.3389/fimmu.2023.1291534. eCollection 2023. Front Immunol. 2023. PMID: 38149243 Free PMC article.
References
-
- Rodriguez-Morales A.J., Cardona-Ospina J.A., Gutiérrez-Ocampo E., Villamizar-Peña R., Holguin-Rivera Y., Escalera-Antezana J.P., Alvarado-Arnez L.E., Bonilla-Aldana D.K., Franco-Paredes C., Henao-Martinez A.F., et al. Clinical, laboratory and imaging features of COVID-19: A systematic review and meta-analysis. Travel Med. Infect. Dis. 2020;34:101623. doi: 10.1016/j.tmaid.2020.101623. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

