TRPM Channels in Human Diseases
- PMID: 33291725
- PMCID: PMC7761947
- DOI: 10.3390/cells9122604
TRPM Channels in Human Diseases
Abstract
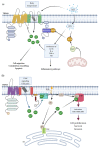
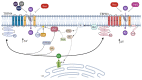
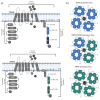
The transient receptor potential melastatin (TRPM) subfamily belongs to the TRP cation channels family. Since the first cloning of TRPM1 in 1989, tremendous progress has been made in identifying novel members of the TRPM subfamily and their functions. The TRPM subfamily is composed of eight members consisting of four six-transmembrane domain subunits, resulting in homomeric or heteromeric channels. From a structural point of view, based on the homology sequence of the coiled-coil in the C-terminus, the eight TRPM members are clustered into four groups: TRPM1/M3, M2/M8, M4/M5 and M6/M7. TRPM subfamily members have been involved in several physiological functions. However, they are also linked to diverse pathophysiological human processes. Alterations in the expression and function of TRPM subfamily ion channels might generate several human diseases including cardiovascular and neurodegenerative alterations, organ dysfunction, cancer and many other channelopathies. These effects position them as remarkable putative targets for novel diagnostic strategies, drug design and therapeutic approaches. Here, we review the current knowledge about the main characteristics of all members of the TRPM family, focusing on their actions in human diseases.
Keywords: TRPM channels; human diseases; ion channels.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

