pH Mapping of Skeletal Muscle by Chemical Exchange Saturation Transfer (CEST) Imaging
- PMID: 33291803
- PMCID: PMC7762073
- DOI: 10.3390/cells9122610
pH Mapping of Skeletal Muscle by Chemical Exchange Saturation Transfer (CEST) Imaging
Abstract
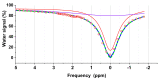
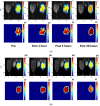
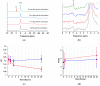
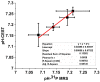
Magnetic resonance imaging (MRI) is extensively used in clinical and basic biomedical research. However, MRI detection of pH changes still poses a technical challenge. Chemical exchange saturation transfer (CEST) imaging is a possible solution to this problem. Using saturation transfer, alterations in the exchange rates between the solute and water protons because of small pH changes can be detected with greater sensitivity. In this study, we examined a fatigued skeletal muscle model in electrically stimulated mice. The measured CEST signal ratio was between 1.96 ppm and 2.6 ppm in the z-spectrum, and this was associated with pH values based on the ratio between the creatine (Cr) and the phosphocreatine (PCr). The CEST results demonstrated a significant contrast change at the electrical stimulation site. Moreover, the pH value was observed to decrease from 7.23 to 7.15 within 20 h after electrical stimulation. This pH decrease was verified by 31P magnetic resonance spectroscopy and behavioral tests, which showed a consistent variation over time.
Keywords: CEST; MRI; muscle; pH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

