Autophagy and Intracellular Membrane Trafficking Subversion by Pathogenic Yersinia Species
- PMID: 33291818
- PMCID: PMC7762052
- DOI: 10.3390/biom10121637
Autophagy and Intracellular Membrane Trafficking Subversion by Pathogenic Yersinia Species
Abstract
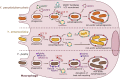
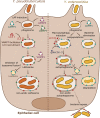
Yersinia pseudotuberculosis, Y. enterocolitica and Y. pestis are pathogenic bacteria capable of causing disease in humans by growing extracellularly in lymph nodes and during systemic infections. While the capacity of these bacteria to invade, replicate, and survive within host cells has been known for long, it is only in recent years that their intracellular stages have been explored in more detail. Current evidence suggests that pathogenic Yersinia are capable of activating autophagy in both phagocytic and epithelial cells, subverting autophagosome formation to create a niche supporting bacterial intracellular replication. In this review, we discuss recent results opening novel perspectives to the understanding of intimate host-pathogens interactions taking place during enteric yersiniosis and plague.
Keywords: Y. pestis; Y. pseudotuberculosis; Y. ruckeri; Yersinia enterocolitica; autophagy; enteric yersiniosis; plague.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
[Pathogenic properties of Yersinia and their role in yersiniosis pathology].Zh Mikrobiol Epidemiol Immunobiol. 1987 Feb;(2):108-15. Zh Mikrobiol Epidemiol Immunobiol. 1987. PMID: 3554847 Review. Russian. No abstract available.
-
YopJ-induced caspase-1 activation in Yersinia-infected macrophages: independent of apoptosis, linked to necrosis, dispensable for innate host defense.PLoS One. 2012;7(4):e36019. doi: 10.1371/journal.pone.0036019. Epub 2012 Apr 26. PLoS One. 2012. PMID: 22563435 Free PMC article.
-
Yersinia pseudotuberculosis and Yersinia pestis are more resistant to bactericidal cationic peptides than Yersinia enterocolitica.Microbiology (Reading). 1998 Jun;144 ( Pt 6):1509-1515. doi: 10.1099/00221287-144-6-1509. Microbiology (Reading). 1998. PMID: 9639921
-
Turning Yersinia pathogenesis outside in: subversion of macrophage function by intracellular yersiniae.Clin Immunol. 2005 Mar;114(3):216-26. doi: 10.1016/j.clim.2004.07.013. Clin Immunol. 2005. PMID: 15721832 Review.
-
Yersinia enterocolitica, a primary model for bacterial invasiveness.Rev Infect Dis. 1987 Jan-Feb;9(1):64-87. doi: 10.1093/clinids/9.1.64. Rev Infect Dis. 1987. PMID: 3547579 Review.
Cited by
-
The battle between bacterial infection and autophagy in aquatic animals.Front Immunol. 2025 Jun 23;16:1614182. doi: 10.3389/fimmu.2025.1614182. eCollection 2025. Front Immunol. 2025. PMID: 40625732 Free PMC article. Review.
-
To eat or not to eat mitochondria? How do host cells cope with mitophagy upon bacterial infection?PLoS Pathog. 2023 Jul 6;19(7):e1011471. doi: 10.1371/journal.ppat.1011471. eCollection 2023 Jul. PLoS Pathog. 2023. PMID: 37410705 Free PMC article. Review.
-
LC3-Associated Phagocytosis in Bacterial Infection.Pathogens. 2022 Jul 30;11(8):863. doi: 10.3390/pathogens11080863. Pathogens. 2022. PMID: 36014984 Free PMC article. Review.
-
Breaking the cellular defense: the role of autophagy evasion in Francisella virulence.Front Cell Infect Microbiol. 2024 Dec 24;14:1523597. doi: 10.3389/fcimb.2024.1523597. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 39776438 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

