Chitosan and Lecithin Ameliorate Osteoarthritis Symptoms Induced by Monoiodoacetate in a Rat Model
- PMID: 33291821
- PMCID: PMC7730914
- DOI: 10.3390/molecules25235738
Chitosan and Lecithin Ameliorate Osteoarthritis Symptoms Induced by Monoiodoacetate in a Rat Model
Abstract
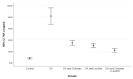
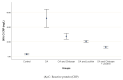
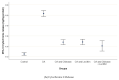
The present work aimed to assess the chondroprotective influence of chitosan and lecithin in a monoiodoacetate (MIA)-induced experimental osteoarthritis (OA) model. Forty male rats weighing 180-200 g were randomly distributed among the following five experimental groups (eight per group): control, MIA-induced OA, MIA-induced OA + chitosan, MIA-induced OA + lecithin, and MIA-induced OA + chitosan + lecithin. The levels of TNF-α, IL6, RF, ROS, and CRP, as well as mitochondrial markers such as mitochondrial swelling, cytochrome C oxidase (complex IV), MMP, and serum oxidative/antioxidant status (MDA level) (MPO and XO activities) were elevated in MIA-induced OA. Also, SDH (complex II) activity in addition to the levels of ATP, glutathione (GSH), and thiol was markedly diminished in the MIA-induced OA group compared to in control rats. These findings show that mitochondrial function is associated with OA pathophysiology and suggest that chitosan and lecithin could be promising potential ameliorative agents in OA animal models. Lecithin was more effective than chitosan in ameliorating all of the abovementioned parameters.
Keywords: chitosan; lecithin; mitochondria; osteoarthritis; pathophysiology; rats.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








Similar articles
-
Synthesis of N,N'-bis(1,5-dimethyl-2-phenyl-1,2-dihydro-3-oxopyrazol-4-yl) sebacamide that ameliorate osteoarthritis symptoms and improve bone marrow matrix structure and cartilage alterations induced by monoiodoacetate in the rat model: "Suggested potent anti-inflammatory agent against COVID-19".Hum Exp Toxicol. 2021 Feb;40(2):325-341. doi: 10.1177/0960327120945779. Epub 2020 Aug 25. Hum Exp Toxicol. 2021. PMID: 32840387 Free PMC article.
-
Cartilage protective and anti-analgesic effects of ALM16 on monosodium iodoacetate induced osteoarthritis in rats.BMC Complement Altern Med. 2019 Nov 21;19(1):325. doi: 10.1186/s12906-019-2746-7. BMC Complement Altern Med. 2019. PMID: 31752825 Free PMC article.
-
Vitamin E ameliorates alterations to the articular cartilage of knee joints induced by monoiodoacetate and diabetes mellitus in rats.Ultrastruct Pathol. 2019;43(2-3):126-134. doi: 10.1080/01913123.2019.1627446. Epub 2019 Jun 9. Ultrastruct Pathol. 2019. PMID: 31177887
-
Silymarin potentiates the anti-inflammatory effects of Celecoxib on chemically induced osteoarthritis in rats.Phytomedicine. 2012 Oct 15;19(13):1200-5. doi: 10.1016/j.phymed.2012.07.008. Epub 2012 Aug 25. Phytomedicine. 2012. PMID: 22925727
-
The Pharmacology of Pain Associated With the Monoiodoacetate Model of Osteoarthritis.Front Pharmacol. 2019 Sep 18;10:974. doi: 10.3389/fphar.2019.00974. eCollection 2019. Front Pharmacol. 2019. PMID: 31619987 Free PMC article. Review.
Cited by
-
Evaluation of the Antiparasitic, Antihepatotoxicity, and Antioxidant Efficacy of Quercetin and Chitosan, Either Alone or in Combination, against Infection Induced by Giardia lamblia in Male Rats.Life (Basel). 2023 Dec 10;13(12):2316. doi: 10.3390/life13122316. Life (Basel). 2023. PMID: 38137916 Free PMC article.
-
Novel insights into the role of metabolic disorder in osteoarthritis.Front Endocrinol (Lausanne). 2024 Dec 18;15:1488481. doi: 10.3389/fendo.2024.1488481. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 39744183 Free PMC article. Review.
-
Proteomic Analyses Reveals the Mechanism of Acupotomy Intervention on the Treatment of Knee Osteoarthritis in Rabbits.Evid Based Complement Alternat Med. 2022 Nov 17;2022:5698387. doi: 10.1155/2022/5698387. eCollection 2022. Evid Based Complement Alternat Med. 2022. PMID: 36437834 Free PMC article.
-
The Potential Benefic Effect of Nicotinamide Riboside in Treating a Murine Model of Monoiodoacetate-Induced Knee Osteoarthritis.J Clin Med. 2023 Nov 3;12(21):6920. doi: 10.3390/jcm12216920. J Clin Med. 2023. PMID: 37959383 Free PMC article.
-
Chitosan-based formulations for therapeutic applications. A recent overview.J Biomed Sci. 2025 Jul 8;32(1):62. doi: 10.1186/s12929-025-01161-7. J Biomed Sci. 2025. PMID: 40629425 Free PMC article. Review.
References
-
- Farrell M., Gibson S., McMeeken J., Helme R. Pain and hyperalgesia in osteoarthritis of the hands. J. Rheumatol. 2000;27:441–447. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

