Autoimmune hemolytic anemia: current knowledge and perspectives
- PMID: 33292368
- PMCID: PMC7677104
- DOI: 10.1186/s12979-020-00208-7
Autoimmune hemolytic anemia: current knowledge and perspectives
Abstract
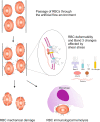
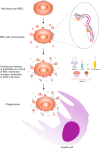
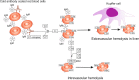
Autoimmune hemolytic anemia (AIHA) is an acquired, heterogeneous group of diseases which includes warm AIHA, cold agglutinin disease (CAD), mixed AIHA, paroxysmal cold hemoglobinuria and atypical AIHA. Currently CAD is defined as a chronic, clonal lymphoproliferative disorder, while the presence of cold agglutinins underlying other diseases is known as cold agglutinin syndrome. AIHA is mediated by autoantibodies directed against red blood cells (RBCs) causing premature erythrocyte destruction. The pathogenesis of AIHA is complex and still not fully understood. Recent studies indicate the involvement of T and B cell dysregulation, reduced CD4+ and CD25+ Tregs, increased clonal expansions of CD8 + T cells, imbalance of Th17/Tregs and Tfh/Tfr, and impaired lymphocyte apoptosis. Changes in some RBC membrane structures, under the influence of mechanical stimuli or oxidative stress, may promote autohemolysis. The clinical presentation and treatment of AIHA are influenced by many factors, including the type of AIHA, degree of hemolysis, underlying diseases, presence of concomitant comorbidities, bone marrow compensatory abilities and the presence of fibrosis and dyserthropoiesis. The main treatment for AIHA is based on the inhibition of autoantibody production by mono- or combination therapy using GKS and/or rituximab and, rarely, immunosuppressive drugs or immunomodulators. Reduction of erythrocyte destruction via splenectomy is currently the third line of treatment for warm AIHA. Supportive treatment including vitamin supplementation, recombinant erythropoietin, thrombosis prophylaxis and the prevention and treatment of infections is essential. New groups of drugs that inhibit immune responses at various levels are being developed intensively, including inhibition of antibody-mediated RBCs phagocytosis, inhibition of B cell and plasma cell frequency and activity, inhibition of IgG recycling, immunomodulation of T lymphocytes function, and complement cascade inhibition. Recent studies have brought about changes in classification and progress in understanding the pathogenesis and treatment of AIHA, although there are still many issues to be resolved, particularly concerning the impact of age-associated changes to immunity.
Keywords: Autoimmune hemolytic anemia; Cold agglutinin disease; Microvesicles; Pathogenesis; Shear stress; Treatment.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

