Clinical management of patients with Cushing syndrome treated with mifepristone: consensus recommendations
- PMID: 33292727
- PMCID: PMC7596972
- DOI: 10.1186/s40842-020-00105-4
Clinical management of patients with Cushing syndrome treated with mifepristone: consensus recommendations
Abstract
Background: While surgery is the first-line treatment for patients with endogenous hypercortisolism (Cushing syndrome [CS]), mifepristone has been shown to be a beneficial medical treatment option, as demonstrated in the SEISMIC (Study of the Efficacy and Safety of Mifepristone in the Treatment of Endogenous Cushing Syndrome) trial. Mifepristone is a competitive glucocorticoid receptor antagonist and progesterone receptor antagonist that is associated with several treatment effects and adverse events that clinicians need to be aware of when considering its use. The objective of this review was to provide updated clinical management recommendations for patients with CS treated with mifepristone.
Methods: A panel of endocrinologists from the US with extensive experience in treating patients with CS, including with mifepristone, convened as part of a clinical advisory board to develop a consensus on the practical, real-world clinical management of patients on mifepristone.
Results: Comprehensive considerations and recommendations are provided for managing mifepristone-associated effects, including symptoms of cortisol withdrawal, hypokalemia, and change in thyroid function; effects related to its antiprogesterone activity; and rash. Additional management strategies to address concomitant medications and special clinical situations, such as surgery and use in specific populations, are also provided.
Conclusion: Safe and effective use of mifepristone requires clinical judgment and close patient monitoring to ensure optimal clinical outcomes. These consensus recommendations provide useful, practical guidance to clinicians using mifepristone.
Keywords: Cushing syndrome; Drug effects; Education.
Conflict of interest statement
D.R.B has been a consultant/advisor for Corcept and a speaker for Corcept, Merck, and Novo Nordisk.
H.E.E. has been a consultant/advisor for Amgen and Corcept, a speaker for Amgen, BI/Lilly, and Corcept, and has received research support from Acasti and Corcept.
B.S.E. has been a consultant/advisor for Corcept.
M.B.G. has been a consultant/advisor for Corcept and Novo Nordisk, and has received research support from Camurus, Corcept, Crinetics, Ionis, Ipsen, Novartis, Novo Nordisk, Opko, Teva, and Strongbridge.
E.E.K. has been a consultant/advisor and speaker for Corcept.
L.A.K. has been a consultant/advisor for Corcept and a speaker for Corcept, Ipsen, and Strongbridge.
B.S. reports that Optime Care, Inc. is the limited distribution contracted pharmacy for Corcept Therapeutics to dispense mifepristone.
S.L.S. has received research support from Corcept and Novartis.
K.C.J.Y. has been a consultant/advisor for Corcept, Novartis, and Strongbridge, and has received research support from Corcept, Crinetics, Millendo, and Novartis.
H.Y. has been a consultant/advisor and a speaker for Corcept.
All authors, except Dr. Salke, received a consulting fee from Corcept for participating in the Consensus Advisory Board in May 2019. Discussion during this event contributed to these consensus recommendations.
Figures


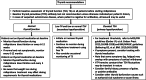
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

