The PLANES study: a protocol for a randomised controlled feasibility study of the placental growth factor (PlGF) blood test-informed care versus standard care alone for women with a small for gestational age fetus at or after 32 + 0 weeks' gestation
- PMID: 33292754
- PMCID: PMC7677818
- DOI: 10.1186/s40814-020-00722-x
The PLANES study: a protocol for a randomised controlled feasibility study of the placental growth factor (PlGF) blood test-informed care versus standard care alone for women with a small for gestational age fetus at or after 32 + 0 weeks' gestation
Abstract
Background: Stillbirth remains a major concern across the globe and in some high-resource countries, such as the UK; efforts to reduce the rate have achieved only modest reductions. One third of stillborn babies are small for gestational age (SGA), and these pregnancies are also at risk of neonatal adverse outcomes and lifelong health problems, especially when delivered preterm. Current UK clinical guidance advocates regular monitoring and early term delivery of the SGA fetus; however, the most appropriate regimen for surveillance of these babies remains unclear and often leads to increased intervention for a large number of these women. This pilot trial will determine the feasibility of a large-scale trial refining the risk of adverse pregnancy outcome in SGA pregnancies using biomarkers of placental function sFlt-1/PlGF, identifying and intervening in only those deemed at highest risk of stillbirth.
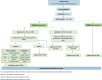
Methods: PLANES is a randomised controlled feasibility study of women with an SGA fetus that will be conducted at two tertiary care hospitals in the UK. Once identified on ultrasound, women will be randomised into two groups in a 3:1 ratio in favour of sFlt-1/PlGF ratio led management vs standard care. Women with an SGA fetus and a normal sFlt-1/PlGF ratio will have a repeat ultrasound and sFlt-1/PlGF ratio every 2 weeks with planned birth delayed until 40 weeks. In those women with an SGA fetus and an abnormal sFlt-1/PlGF ratio, we will offer birth from 37 weeks or sooner if there are other concerning features on ultrasound. Women assigned to standard care will have an sFlt-1/PlGF ratio taken, but the results will be concealed from the clinical team, and the woman's pregnancy will be managed as per the local NHS hospital policy. This integrated mixed method study will also involve a health economic analysis and a perspective work package exploring trial feasibility through interviews and questionnaires with participants, their partners, and clinicians.
Discussion: Our aim is to determine feasibility through the assessment of our ability to recruit and retain participants to the study. Results from this pilot study will inform the design of a future large randomised controlled trial that will be adequately powered for adverse pregnancy outcome. Such a study would provide the evidence needed to guide future management of the SGA fetus.
Trial registration: ISRCTN58254381 . Registered on 4 July 2019.
Keywords: Fetal growth restriction (FGR); Intrauterine growth restriction; Placenta; Placental growth factor; Small for gestational age (SGA); Soluble fms-like tyrosine kinase.
Conflict of interest statement
No authors report any conflicts of interest.
Similar articles
-
Ophthalmic artery Doppler and biomarkers of impaired placentation at 36 weeks' gestation in pregnancies with small fetuses.Ultrasound Obstet Gynecol. 2024 Mar;63(3):358-364. doi: 10.1002/uog.27521. Epub 2024 Feb 10. Ultrasound Obstet Gynecol. 2024. PMID: 37902727
-
Soluble fms-like tyrosine kinase to placental growth factor ratio in different stages of early-onset fetal growth restriction and small for gestational age.Acta Obstet Gynecol Scand. 2021 Jan;100(1):119-128. doi: 10.1111/aogs.13978. Epub 2020 Sep 14. Acta Obstet Gynecol Scand. 2021. PMID: 32860218
-
Evaluation of angiogenic factors in prediction of growth-related neonatal morbidity at term and comparison with competing-risks model.Ultrasound Obstet Gynecol. 2024 Apr;63(4):457-465. doi: 10.1002/uog.27533. Ultrasound Obstet Gynecol. 2024. PMID: 37963283
-
Biochemical tests of placental function versus ultrasound assessment of fetal size for stillbirth and small-for-gestational-age infants.Cochrane Database Syst Rev. 2019 May 14;5(5):CD012245. doi: 10.1002/14651858.CD012245.pub2. Cochrane Database Syst Rev. 2019. PMID: 31087568 Free PMC article.
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
Cited by
-
Racial differences in the associations between adiposity, placental growth hormone and inflammatory cytokines in pregnant women.Front Endocrinol (Lausanne). 2023 Mar 17;14:1100724. doi: 10.3389/fendo.2023.1100724. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37025401 Free PMC article.
-
Maternal PlGF and umbilical Dopplers predict pregnancy outcomes at diagnosis of early-onset fetal growth restriction.J Clin Invest. 2023 Sep 15;133(18):e169199. doi: 10.1172/JCI169199. J Clin Invest. 2023. PMID: 37712421 Free PMC article.
-
The role of the PLGF in the prediction of the outcome in pregnancies with a small for gestational age fetus.Arch Gynecol Obstet. 2024 Jul;310(1):237-243. doi: 10.1007/s00404-023-07214-2. Epub 2023 Oct 14. Arch Gynecol Obstet. 2024. PMID: 37837546
References
-
- Sharp AN, Alfirevic Z. First trimester screening can predict adverse pregnancy outcomes. Prenat Diagn. 2014;34(7):660–7. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous