An observational study of the reactogenicity and immunogenicity of 13-valent pneumococcal conjugate vaccine in women of childbearing age in Papua New Guinea
- PMID: 33292822
- PMCID: PMC7687988
- DOI: 10.1186/s41479-020-00076-1
An observational study of the reactogenicity and immunogenicity of 13-valent pneumococcal conjugate vaccine in women of childbearing age in Papua New Guinea
Abstract
Background: Maternal immunization with pneumococcal conjugate vaccine (PCV) may protect young infants in high-risk settings against the high risk of pneumococcal infections in early life. The aim of this study was to determine the safety and immunogenicity of 13-valent PCV (PCV13) in healthy women of childbearing age in PNG.
Methods: As part of this observational study, 50 non-pregnant women of childbearing age (18-45 yrs. old) living in the highlands of PNG were vaccinated with a single dose of PCV13. Local and systemic reactogenicity were assessed 24-48 h after vaccination. Venous blood samples were collected before and 1 month after vaccination to measure PCV13 serotype-specific IgG antibody concentrations.
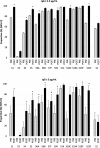
Results: No severe adverse effects were reported during the 1-month follow-up period. IgG antibody concentrations significantly increased after vaccination for all PCV13 serotypes. One month after vaccination IgG antibody levels ≥2.5 μg/mL were reached in at least 75% of women for all PCV13 serotypes, except serotype 3, and ≥ 5 μg/mL in at least 75% of women for 7 serotypes (serotypes 6B, 9 V, 14, 18C, 19A, 19F and 23F).
Conclusion: PCV13 is safe and immunogenic in women of childbearing age living in a high-risk setting in PNG. This supports the implementation of studies to investigate the safety and immunogenicity of maternal PCV vaccination in high-risk settings as a strategy to protect infants in these settings against the high risk of pneumococcal infections in early life.
Trial registration: NCT04183322 . Registered 3 December 2019 - Retrospectively registered.
Keywords: Adults; Childbearing age; Immunogenicity; Non-pregnant; PCV; Papua New Guinea; Pneumococcal; Pneumococcal conjugate vaccine; Safety; Vaccine; WOCBA.
Conflict of interest statement
DL has received support from Pfizer Australia to attend conferences, an honorarium from Merck Vaccines to give a seminar at their offices in Pennsylvania and support from Merck Vaccines to attend a conference; and is an investigator on an investigator-initiated research grant that was funded by Pfizer Australia. PR has received non-financial support from Pfizer, grants from GlaxoSmithKline, grants from Pfizer, and non-financial support from GlaxoSmithKline for work outside the submitted work. WP and AVDB have received funding from Pfizer Australia to attend research conferences. AVDB has conducted contract work for Pfizer Inc. as a consultant with P95 Epidemiology and Pharmacovigilance outside the scope and independent of the submitted work.
Figures
References
-
- O'Brien KL, Wolfson LJ, Watt JP, Henkle E, Deloria-Knoll M, McCall N, et al. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet. 2009;374(9693):893–902. - PubMed
-
- Bogaert D, De Groot R, Hermans PW. Streptococcus pneumoniae colonisation: the key to pneumococcal disease. Lancet Infect Dis. 2004;4(3):144–154. - PubMed
-
- Simell B, Auranen K, Kayhty H, Goldblatt D, Dagan R, O'Brien KL, et al. The fundamental link between pneumococcal carriage and disease. Expert Rev Vaccines. 2012;11(7):841–855. - PubMed
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

