Enhancing community participation for stroke survivors with cognitive impairment: study protocol for a randomised controlled trial in Taiwan
- PMID: 33293312
- PMCID: PMC7722819
- DOI: 10.1136/bmjopen-2020-040241
Enhancing community participation for stroke survivors with cognitive impairment: study protocol for a randomised controlled trial in Taiwan
Abstract
Introduction: Stroke can lead to life-long disability and constitutes a huge financial burden on the family and society. Stroke survivors with cognitive impairment often experience considerable challenges in the process of recovery and returning to society. Interventions that effectively help individuals resume essential daily activities and return to active participation in their communities are lacking. This study examines the efficacy of a newly-developed intervention programme, the Optimising Participation after Stroke through Strategy-training (OPASS) programme, for improving community participation among stroke survivors with cognitive impairment.
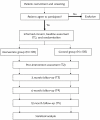
Methods and analysis: A single-blind, parallel-group randomised controlled trial with allocation concealment and assessor blinding will be implemented to assess the efficacy of the OPASS programme. An expected 210 adults with cognitive impairment following stroke will be randomly assigned to either the experimental intervention (OPASS) group or the attention control group. In addition to their usual rehabilitation, both groups will receive 45 min sessions, twice weekly for a total of 12-15 sessions. The primary outcome is change in participation performance, which will be measured using the participation measure-three domains, four dimensions scale. Additional measures include the Activity Measure for Post-Acute Care generic outpatient short forms, Montreal Cognitive Assessment, Stroop Test, Trail Making Test and General Self-Efficacy Scale. These scales will be administered at baseline, post-intervention, 3-month follow-up, 6-month follow-up and 12-month follow-up. Their results will be analysed using multiple linear regression models and mixed-effects regression models. Further assessment of feasibility and acceptability of the intervention will be conducted through structured interviews with participants, caregivers and therapists. These interviews will be transcribed and thematically analysed.
Ethics and dissemination: Ethics approval was obtained from the Ethics Committee of Taipei Medical University (approval number: N201804055). The findings will be disseminated through presentations at scientific conferences and through publication in peer-reviewed journals.
Trial registration number: NCT03792061; pre-results.
Keywords: cognitive dysfunction; rehabilitation; social participation; stroke.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures

References
-
- World Health Organization The atlas of heart disease and stroke 2015. Available: http://www.who.int/cardiovascular_diseases/resources/atlas/en/ [Accessed 23 September 2015].
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
