Active Transition of Fear Memory Phase from Reconsolidation to Extinction through ERK-Mediated Prevention of Reconsolidation
- PMID: 33293359
- PMCID: PMC7888227
- DOI: 10.1523/JNEUROSCI.1854-20.2020
Active Transition of Fear Memory Phase from Reconsolidation to Extinction through ERK-Mediated Prevention of Reconsolidation
Abstract
The retrieval of fear memory induces two opposite memory process, i.e., reconsolidation and extinction. Brief retrieval induces reconsolidation to maintain or enhance fear memory, while prolonged retrieval extinguishes this memory. Although the mechanisms of reconsolidation and extinction have been investigated, it remains unknown how fear memory phases are switched from reconsolidation to extinction during memory retrieval. Here, we show that an extracellular signal-regulated kinase (ERK)-dependent memory transition process after retrieval regulates the switch of memory phases from reconsolidation to extinction by preventing induction of reconsolidation in an inhibitory avoidance (IA) task in male mice. First, the transition memory phase, which cancels the induction of reconsolidation, but is insufficient for the acquisition of extinction, was identified after reconsolidation, but before extinction phases. Second, the reconsolidation, transition, and extinction phases after memory retrieval showed distinct molecular and cellular signatures through cAMP responsive element binding protein (CREB) and ERK phosphorylation in the amygdala, hippocampus, and medial prefrontal cortex (mPFC). The reconsolidation phase showed increased CREB phosphorylation, while the extinction phase displayed several neural populations with various combinations of CREB and/or ERK phosphorylation, in these brain regions. Interestingly, the three memory phases, including the transition phase, showed transient ERK activation immediately after retrieval. Most importantly, the blockade of ERK in the amygdala, hippocampus, or mPFC at the transition memory phase disinhibited reconsolidation-induced enhancement of IA memory. These observations suggest that the ERK-signaling pathway actively regulates the transition of memory phase from reconsolidation to extinction and this process functions as a switch that cancels reconsolidation of fear memory.SIGNIFICANCE STATEMENT Retrieval of fear memory induces two opposite memory process; reconsolidation and extinction. Reconsolidation maintains/enhances fear memory, while extinction weakens fear memory. It remains unknown how memory phases are switched from reconsolidation to extinction during retrieval. Here, we identified an active memory transition process functioning as a switch that inhibits reconsolidation. This memory transition phase showed a transient increase of extracellular signal-regulated kinase (ERK) phosphorylation in the amygdala, hippocampus and medial prefrontal cortex (mPFC). Interestingly, inhibition of ERK in these regions at the transition phase disinhibited the reconsolidation-mediated enhancement of inhibitory avoidance (IA) memory. These findings suggest that the transition memory process actively regulates the switch of fear memory phases of fear memory by preventing induction of reconsolidation through the activation of the ERK-signaling pathway.
Keywords: ERK; extinction; fear memory; reconsolidation; transition.
Copyright © 2021 the authors.
Figures
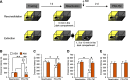


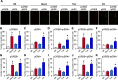
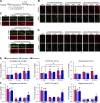
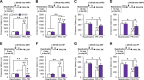

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous
