Calmodulin Bidirectionally Regulates Evoked and Spontaneous Neurotransmitter Release at Retinal Ribbon Synapses
- PMID: 33293457
- PMCID: PMC7808332
- DOI: 10.1523/ENEURO.0257-20.2020
Calmodulin Bidirectionally Regulates Evoked and Spontaneous Neurotransmitter Release at Retinal Ribbon Synapses
Abstract
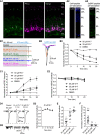
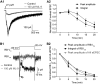
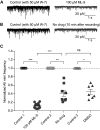
For decades, a role for the Ca2+-binding protein calmodulin (CaM) in Ca2+-dependent presynaptic modulation of synaptic transmission has been recognized. Here, we investigated the influence of CaM on evoked and spontaneous neurotransmission at rod bipolar (RB) cell→AII amacrine cell synapses in the mouse retina. Our work was motivated by the observations that expression of CaM in RB axon terminals is extremely high and that [Ca2+] in RB terminals normally rises sufficiently to saturate endogenous buffers, making tonic CaM activation likely. Taking advantage of a model in which RBs can be stimulated by expressed channelrhodopsin-2 (ChR2) to avoid dialysis of the presynaptic terminal, we found that inhibition of CaM dramatically decreased evoked release by inhibition of presynaptic Ca channels while at the same time potentiating both Ca2+-dependent and Ca2+-independent spontaneous release. Remarkably, inhibition of myosin light chain kinase (MLCK), but not other CaM-dependent targets, mimicked the effects of CaM inhibition on evoked and spontaneous release. Importantly, initial antagonism of CaM occluded the effect of subsequent inhibition of MLCK on spontaneous release. We conclude that CaM, by acting through MLCK, bidirectionally regulates evoked and spontaneous release at retinal ribbon synapses.
Keywords: AII amacrine cell; calmodulin; myosin light chain kinase; neurotransmitter release; retinal rod bipolar cell; ribbon synapse.
Copyright © 2021 Liang et al.
Figures






Similar articles
-
Multiple Calcium Channel Types with Unique Expression Patterns Mediate Retinal Signaling at Bipolar Cell Ribbon Synapses.J Neurosci. 2022 Aug 24;42(34):6487-6505. doi: 10.1523/JNEUROSCI.0183-22.2022. Epub 2022 Jul 27. J Neurosci. 2022. PMID: 35896423 Free PMC article.
-
Efficient stimulus-secretion coupling at ribbon synapses requires RIM-binding protein tethering of L-type Ca2+ channels.Proc Natl Acad Sci U S A. 2017 Sep 19;114(38):E8081-E8090. doi: 10.1073/pnas.1702991114. Epub 2017 Sep 5. Proc Natl Acad Sci U S A. 2017. PMID: 28874522 Free PMC article.
-
RIM1/2-Mediated Facilitation of Cav1.4 Channel Opening Is Required for Ca2+-Stimulated Release in Mouse Rod Photoreceptors.J Neurosci. 2015 Sep 23;35(38):13133-47. doi: 10.1523/JNEUROSCI.0658-15.2015. J Neurosci. 2015. PMID: 26400943 Free PMC article.
-
Neurotransmitter release at ribbon synapses in the retina.Immunol Cell Biol. 2000 Aug;78(4):442-6. doi: 10.1046/j.1440-1711.2000.00923.x. Immunol Cell Biol. 2000. PMID: 10947871 Review.
-
Presynaptic proteins of ribbon synapses in the retina.Microsc Res Tech. 2000 Jul 15;50(2):141-50. doi: 10.1002/1097-0029(20000715)50:2<141::AID-JEMT6>3.0.CO;2-B. Microsc Res Tech. 2000. PMID: 10891878 Review.
Cited by
-
Multiple Calcium Channel Types with Unique Expression Patterns Mediate Retinal Signaling at Bipolar Cell Ribbon Synapses.J Neurosci. 2022 Aug 24;42(34):6487-6505. doi: 10.1523/JNEUROSCI.0183-22.2022. Epub 2022 Jul 27. J Neurosci. 2022. PMID: 35896423 Free PMC article.
-
Identification of TPBG-Expressing Amacrine Cells in DAT-tdTomato Mouse.Invest Ophthalmol Vis Sci. 2022 May 2;63(5):13. doi: 10.1167/iovs.63.5.13. Invest Ophthalmol Vis Sci. 2022. PMID: 35551574 Free PMC article.
-
Retinoic Acid-Dependent Loss of Synaptic Output from Bipolar Cells Impairs Visual Information Processing in Inherited Retinal Degeneration.J Neurosci. 2024 Aug 28;44(35):e0129242024. doi: 10.1523/JNEUROSCI.0129-24.2024. J Neurosci. 2024. PMID: 39060177 Free PMC article.
-
Glutamate Transporters EAAT2 and EAAT5 Differentially Shape Synaptic Transmission from Rod Bipolar Cell Terminals.eNeuro. 2022 May 18;9(3):ENEURO.0074-22.2022. doi: 10.1523/ENEURO.0074-22.2022. Print 2022 May-Jun. eNeuro. 2022. PMID: 35523583 Free PMC article.
-
The initiator of neuroexcitotoxicity and ferroptosis in ischemic stroke: Glutamate accumulation.Front Mol Neurosci. 2023 Mar 23;16:1113081. doi: 10.3389/fnmol.2023.1113081. eCollection 2023. Front Mol Neurosci. 2023. PMID: 37033381 Free PMC article. Review.
References
-
- Abrahamsson T, Chou CYC, Li SY, Mancino A, Costa RP, Brock JA, Nuro E, Buchanan KA, Elgar D, Blackman AV, Tudor-Jones A, Oyrer J, Farmer WT, Murai KK, Sjöström PJ (2017) Differential regulation of evoked and spontaneous release by presynaptic NMDA receptors. Neuron 96:839–855. 10.1016/j.neuron.2017.09.030 - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
