Mesenchymal VEGFA induces aberrant differentiation in heterotopic ossification
- PMID: 31840004
- PMCID: PMC6904752
- DOI: 10.1038/s41413-019-0075-6
Mesenchymal VEGFA induces aberrant differentiation in heterotopic ossification
Abstract
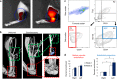
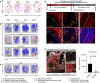
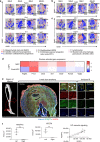
Heterotopic ossification (HO) is a debilitating condition characterized by the pathologic formation of ectopic bone. HO occurs commonly following orthopedic surgeries, burns, and neurologic injuries. While surgical excision may provide palliation, the procedure is often burdened with significant intra-operative blood loss due to a more robust contribution of blood supply to the pathologic bone than to native bone. Based on these clinical observations, we set out to examine the role of vascular signaling in HO. Vascular endothelial growth factor A (VEGFA) has previously been shown to be a crucial pro-angiogenic and pro-osteogenic cue during normal bone development and homeostasis. Our findings, using a validated mouse model of HO, demonstrate that HO lesions are highly vascular, and that VEGFA is critical to ectopic bone formation, despite lacking a contribution of endothelial cells within the developing anlagen.
Keywords: Bone; Pathogenesis.
© The Author(s) 2019.
Conflict of interest statement
Competing interestsA.W.J. serves on the scientific advisory board for Novadip LLC and also receives laboratory financial support from MTF Biologics for research unrelated to the current project. All other authors declare no competing interests..
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources

