Absence of relevant QT interval prolongation in not critically ill COVID-19 patients
- PMID: 33293554
- PMCID: PMC7722753
- DOI: 10.1038/s41598-020-78360-9
Absence of relevant QT interval prolongation in not critically ill COVID-19 patients
Abstract
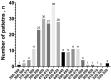
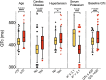
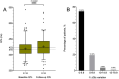
SARS-CoV-2 is a rapidly evolving pandemic causing great morbimortality. Medical therapy with hydroxicloroquine, azitromycin and protease inhibitors is being empirically used, with reported data of QTc interval prolongation. Our aim is to assess QT interval behaviour in a not critically ill and not monitored cohort of patients. We evaluated admitted and ambulatory patients with COVID-19 patients with 12 lead electrocardiogram at 48 h after treatment initiation. Other clinical and analytical variables were collected. Statistical analysis was performed to assess the magnitude of the QT interval prolongation under treatment and to identify clinical, analytical and electrocardiographic risk markers of QT prolongation independent predictors. We included 219 patients (mean age of 63.6 ± 17.4 years, 48.9% were women and 16.4% were outpatients. The median baseline QTc was 416 ms (IQR 404-433), and after treatment QTc was prolonged to 423 ms (405-438) (P < 0.001), with an average increase of 1.8%. Most of the patients presented a normal QTc under treatment, with only 31 cases (14.1%) showing a QTc interval > 460 ms, and just one case with QTc > 500 ms. Advanced age, longer QTc basal at the basal ECG and lower potassium levels were independent predictors of QTc interval prolongation. Ambulatory and not critically ill patients with COVID-19 treated with hydroxychloroquine, azithromycin and/or antiretrovirals develop a significant, but not relevant, QT interval prolongation.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

