Multiscale 3D phenotyping of human cerebral organoids
- PMID: 33293587
- PMCID: PMC7723053
- DOI: 10.1038/s41598-020-78130-7
Multiscale 3D phenotyping of human cerebral organoids
Abstract
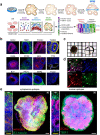
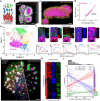
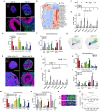
Brain organoids grown from human pluripotent stem cells self-organize into cytoarchitectures resembling the developing human brain. These three-dimensional models offer an unprecedented opportunity to study human brain development and dysfunction. Characterization currently sacrifices spatial information for single-cell or histological analysis leaving whole-tissue analysis mostly unexplored. Here, we present the SCOUT pipeline for automated multiscale comparative analysis of intact cerebral organoids. Our integrated technology platform can rapidly clear, label, and image intact organoids. Algorithmic- and convolutional neural network-based image analysis extract hundreds of features characterizing molecular, cellular, spatial, cytoarchitectural, and organoid-wide properties from fluorescence microscopy datasets. Comprehensive analysis of 46 intact organoids and ~ 100 million cells reveals quantitative multiscale "phenotypes" for organoid development, culture protocols and Zika virus infection. SCOUT provides a much-needed framework for comparative analysis of emerging 3D in vitro models using fluorescence microscopy.
Conflict of interest statement
K.C. is a cofounder of LifeCanvas Technologies, a startup that provides solutions for 3D tissue processing and analysis.
Figures



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

