Development of vaccines and antivirals for combating viral pandemics
- PMID: 33293724
- PMCID: PMC8336060
- DOI: 10.1038/s41551-020-00658-w
Development of vaccines and antivirals for combating viral pandemics
Abstract
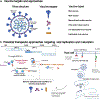
Proactive efforts towards the development of new vaccines and antivirals, and the elimination of bottlenecks in vaccine development, will be essential to containing and eradicating future pandemics.
Conflict of interest statement
Competing interests
The authors are named on patents that describe the use of nucleoside-modified mRNA as a platform to deliver therapeutic proteins and vaccines. These competing interests have been fully disclosed to the University of Pennsylvania, and an approved plan for managing any potential conflicts arising from the licensing of the patents is in place.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

