MP-AzeFlu Improves the Quality-of-Life of Patients with Allergic Rhinitis
- PMID: 33293835
- PMCID: PMC7719305
- DOI: 10.2147/JAA.S277734
MP-AzeFlu Improves the Quality-of-Life of Patients with Allergic Rhinitis
Abstract
Purpose: Patients with poorly controlled allergic rhinitis (AR) experience nasal symptoms, sleep disturbances, activity impairment, and decreased quality-of-life (QoL). MP-AzeFlu is safe and effective for moderate-to-severe seasonal and perennial AR, but its impact on QoL requires investigation in the real-world, especially among phenotypes of immunoglobulin (Ig)E-mediated AR. This subanalysis of an observational study evaluated response to MP-AzeFlu via assessment of sleep quality and trouble with daily activities.
Patients and methods: This multicenter, prospective, non-interventional, real-life study included a convenience sample of patients with a history of moderate-to-severe AR presenting with acute AR symptoms (visual analog scale [VAS] ≥50 mm). Over approximately 14 days of treatment with MP-AzeFlu (137 µg azelastine HCL and 50 µg fluticasone propionate administered via single 0.137-mL spray in each nostril twice daily), changes in sleep quality and trouble with daily work, school, social, and outdoor activities were evaluated using a VAS for the entire study population and for four subgroups based on IgE response phenotype. VAS scores ranged from "not at all troubled" (0 mm) to "extremely troubled" (100 mm).
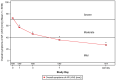
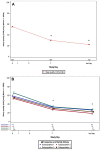
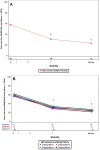
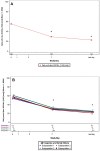
Results: Following MP-AzeFlu treatment, mean VAS scores for sleep quality impairment and work or school impairment decreased from 55.2 mm at baseline to 22.1 mm and 57.6 mm at baseline to 23.0 mm, respectively, after ~14 days. Similar results were observed for mean VAS scores for impairment of social activity (55.1 mm to 22.4 mm) and impairment of outdoor activity (64.4 mm to 25.0 mm). For all VAS scores, results were similar across populations, regardless of phenotype of IgE-mediated disease, comorbidity, age, and sex.
Conclusion: MP-AzeFlu relieves symptoms and improves patient-reported QoL, illustrated by better sleep quality and less impairment of work, school, social, and outdoor activities after 14 days. The QoL benefits of MP-AzeFlu were consistent regardless of the phenotype of IgE-mediated disease.
Registration: Clinical Trial Registration (CTR) Number: EUPAS23075. Trial Register Date: March 12, 2018. First patient visit; Last patient visit: February 2018; April 2019.
Keywords: azelastine hydrochloride; daily activities; fluticasone propionate; sleep.
© 2020 van Weissenbruch et al.
Conflict of interest statement
RVW has nothing to disclose. LK worked as a paid consultant for Allergopharma, MEDA/Mylan, HAL Allergy, ALK Abello, and LET! Pharma; has received financial grants from Allergopharma, ALK Abello, Allergy Therapeutics, Stallergenes, Quintiles, HAL Allergy, LET! Pharma, Sanofi, AstraZeneca, GSK, ASIT Biotech, and Lofarma; reports grants and personal fees from Allergopharma, MEDA/Mylan, and Sanofi; personal fees from HAL Allergy, LETI Pharma, and Allergy Therapeutics; grants from ASIT biotech, ALK Abelló, Stallergenes, Quintiles, Lofarma, AstraZeneca, GSK, and Inmunotek, outside the submitted work; and membership in the following: AeDA, DGHNO, Deutsche Akademie für Allergologie und klinische Immunologie, HNO-BV, GPA, and EAACI. GG was a paid consultant and speaker for Astra-Zeneca, Chiesi, Bristol Myers Squibb, MSD, Berlin Chemi, Boehringer Ingelheim, Roche, Novartis, Pfizer, Orion, including Ipsen, and Mylan as speaker. ME is an employee of MEDA Pharma GmbH & Co. KG (a Mylan Company). AK is a Mylan, Inc. employee and shareholder, an employee of Mylan/MEDA Pharmaceuticals, and has been employed at Novartis and Lundbeck pharmaceutical companies. FK is an employee of MEDA Pharma GmbH & Co. KG (a Mylan Company) and reports personal fees from MEDA Pharma GmbH & Co KG (A Mylan Company) during the conduct of the study and outside the submitted work. DTN is an employee of MEDA Pharma GmbH & Co. KG (a Mylan Company). HCK is an employee of MEDA Pharma GmbH & Co. KG and worked as a paid consultant for AstraZeneca, Boehringer lngelheim, Chiesi, GSK, and Novartis. WP has been a paid speaker for and worked as a paid consultant for AstraZeneca, Boehringer lngelheim, Chiesi, GSK, Novartis, and TEVA, and reports personal fees from AstraZeneca, Chiesi, GSK, Menarini, Boehringer Ingelheim, and Sanofi outside the submitted work. GS has received financial grants from GSK for the mepolizumab study, has worked as a paid consultant and speaker for Meda/Mylan and ALK-Abello, reports personal fees from Mylan and ALK outside the submitted work, and is/was the lead for Allergic Rhinitis education in EUFOREA, lead on BSACI Rhinitis Management guidelines, and scientific chief editor of the Rhinology section of Frontiers in Allergy. DP has board membership with Amgen, AstraZeneca, Boehringer Ingelheim, Chiesi, Circassia, Mylan, Mundipharma, Napp, Novartis, Regeneron Pharmaceuticals, Sanofi Genzyme, and Teva Pharmaceuticals; consultancy agreements with Amgen, AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Mylan, Mundipharma, Napp, Novartis, Pfizer, Teva Pharmaceuticals, and Theravance; grants and unrestricted funding for investigator-initiated studies (conducted through Observational & Pragmatic Research Institute Pte Ltd) from AKL Research and Development Ltd, AstraZeneca, Boehringer Ingelheim, British Lung Foundation, Chiesi, Circassia, Mylan, Mundipharma, Napp, Novartis, Pfizer, Regeneron Pharmaceuticals, Respiratory Effectiveness Group, Sanofi Genzyme, Teva Pharmaceuticals, Theravance, UK National Health Service, and Zentiva (Sanofi Generics); payment for lectures/speaking engagements from AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, GlaxoSmithKline, Kyorin, Mylan, Merck, Mundipharma, Novartis, Pfizer, Regeneron Pharmaceuticals, Sanofi Genzyme, and Teva Pharmaceuticals; payment for manuscript preparation from Mundipharma and Teva Pharmaceuticals; payment for the development of educational materials from Mundipharma and Novartis; payment for travel/accommodation/meeting expenses from AstraZeneca, Boehringer Ingelheim, Circassia, Mundipharma, Napp, Novartis, and Teva Pharmaceuticals; funding for patient enrollment or completion of research from Chiesi, Novartis, Teva Pharmaceuticals, and Zentiva (Sanofi Generics); stock/stock options from AKL Research and Development Ltd, which produces phytopharmaceuticals; owns 74% of the social enterprise Optimum Patient Care Ltd (Australia and United Kingdom) and 74% of Observational & Pragmatic Research Institute Pte Ltd (Singapore); and is peer reviewer for grant committees of the Efficacy and Mechanism Evaluation Programme and Health Technology Assessment. In addition, DP reports personal fees from Amgen, Cipla, GlaxoSmithKline, Kyorin, Merck, and Zentiva (Sanofi Generics), grants from AKL Research and Development Ltd, British Lung Foundation, Respiratory Effectiveness Group, UK National Health Service; grants and personal fees from AstraZeneca, Boehringer Ingelheim, Chiesi, Circassia, Mylan, Mundipharma, Napp, Novartis, Pfizer, Regeneron Pharmaceuticals, Sanofi Genzyme, Teva, and Theravance; and non-financial support from Efficacy and Mechanism Evaluation Programme and Health Technology Assessment outside the submitted work; and owns 5% shareholding in TimeStamp, which develops adherence monitoring technology. JM has conducted research/received research grant support from Mylan/MEDA Pharma, URIACH Group, GSK, MSD, FAES, and UCB; received consultancy fees from Mylan/MEDA Pharma, URIACH Group, Allakos, ALK-Abelló, Genentech–Roche, Novartis, Regeneron, Sanofi Genzyme, GSK, MSD, Hartington Pharmaceuticals, and UCB; was a paid instructor for Novartis; was a speaker for Mylan/MEDA Pharma, URIACH Group, Genentech–Roche, Novartis, Regeneron, Sanofi Genzyme, GSK, MSD, Hartington Pharmaceuticals, UCB, and Glenmark; reports grants and personal fees from URIACH Group; was on the speakers bureau/advisory board for Mylan/MEDA Pharma, Sanofi-Genzyme & Regeneron, and Genentech-Novartis; advisory board for AstraZeneca and GSK; and speakers bureau for MENARINI and MSD outside the submitted work. The authors report no other potential conflicts of interest for this work.
Figures
Similar articles
-
Allergic rhinitis and asthma symptoms in a real-life study of MP-AzeFlu to treat multimorbid allergic rhinitis and asthma.Clin Mol Allergy. 2020 Aug 6;18:15. doi: 10.1186/s12948-020-00130-9. eCollection 2020. Clin Mol Allergy. 2020. PMID: 32782442 Free PMC article.
-
Effect of Specific Immunoglobulin E Response and Comorbidities on Effectiveness of MP-AzeFlu in a Real-Life Study.Int Arch Allergy Immunol. 2020;181(10):754-764. doi: 10.1159/000508749. Epub 2020 Aug 21. Int Arch Allergy Immunol. 2020. PMID: 32829329 Free PMC article.
-
Real-Life Effectiveness of MP-AzeFlu (Dymista®) in Swedish Patients with Persistent Allergic Rhinitis, Assessed by the Visual Analogue Scale.Pragmat Obs Res. 2023 Jan 4;14:1-11. doi: 10.2147/POR.S375403. eCollection 2023. Pragmat Obs Res. 2023. PMID: 36628265 Free PMC article.
-
MP-AzeFlu in Moderate-to-Severe Allergic Rhinitis: A Literature Review.Int Arch Allergy Immunol. 2021;182(11):1026-1035. doi: 10.1159/000516417. Epub 2021 Jun 3. Int Arch Allergy Immunol. 2021. PMID: 34082425
-
The Use of Azelastine Hydrochloride/Fluticasone Propionate in the Management of Allergic Rhinitis in Asia: A Review.J Asthma Allergy. 2024 Jul 12;17:667-679. doi: 10.2147/JAA.S451733. eCollection 2024. J Asthma Allergy. 2024. PMID: 39045291 Free PMC article. Review.
Cited by
-
A Clinical Study to Assess the Efficacy and Safety of MP-AzeFlu Nasal Spray in Comparison to Commercially Available Azelastine Hydrochloride and Fluticasone Propionate Nasal Sprays in Chinese Volunteers with Allergic Rhinitis.Pulm Ther. 2023 Sep;9(3):411-427. doi: 10.1007/s41030-023-00238-8. Epub 2023 Aug 14. Pulm Ther. 2023. PMID: 37580498 Free PMC article.
References
-
- Bousquet J, Khaltaev N, Cruz AA, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen). Allergy. 2008;63(Suppl 86):8–160. - PubMed
-
- Canonica GW, Bousquet J, Mullol J, Scadding GK, Virchow JC. A survey of the burden of allergic rhinitis in Europe. Allergy. 2007;62(Suppl 85):17–25. - PubMed
-
- de la Hoz Caballer B, Rodriguez M, Fraj J, Cerecedo I, Antolín-Amérigo D, Colás C. Allergic rhinitis and its impact on work productivity in primary care practice and a comparison with other common diseases: the cross-sectional study to evaluate work productivity in allergic rhinitis compared with other common diseases (CAPRI) study. Am J Rhinol Allergy. 2012;26(5):390–394. doi:10.2500/ajra.2012.26.3799 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

