Collagen production and niche engineering: A novel strategy for cancer cells to survive acidosis in DCIS and evolve
- PMID: 33294017
- PMCID: PMC7691473
- DOI: 10.1111/eva.13075
Collagen production and niche engineering: A novel strategy for cancer cells to survive acidosis in DCIS and evolve
Abstract
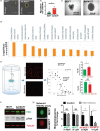
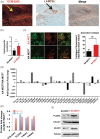
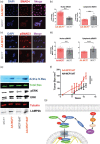
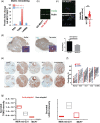
Growing tumors are dynamic and nonlinear ecosystems, wherein cancer cells adapt to their local microenvironment, and these adaptations further modify the environment, inducing more changes. From nascent intraductal neoplasms to disseminated metastatic disease, several levels of evolutionary adaptations and selections occur. Here, we focus on one example of such an adaptation mechanism, namely, "niche construction" promoted by adaptation to acidosis, which is a metabolic adaptation to the early harsh environment in intraductal neoplasms. The avascular characteristics of ductal carcinoma in situ (DCIS) make the periluminal volume profoundly acidic, and cancer cells must adapt to this to survive. Based on discovery proteomics, we hypothesized that a component of acid adaptation involves production of collagen by pre-cancer cells that remodels the extracellular matrix (ECM) and stabilizes cells under acid stress. The proteomic data were surprising as collagen production and deposition are commonly believed to be the responsibility of mesenchymally derived fibroblasts, and not cells of epithelial origin. Subsequent experiments in 3D culture, spinning disk and second harmonic generation microscopy of DCIS lesions in patients' samples are concordant. Collagen production assay by acid-adapted cells in vitro demonstrated that the mechanism of induction involves the RAS and SMAD pathways. Secretome analyses show upregulation of ECM remodeling enzymes such as TGM2 and LOXL2 that are collagen crosslinkers. These data strongly indicate that acidosis in incipient cancers induces collagen production by cancer cells and support the hypothesis that this adaptation initiates a tumor-permissive microenvironment promoting survival and growth of nascent cancers.
Keywords: K‐RAS; Niche construction and engineering; SMAD; anoikis; cancer evolution; collagen production; extracellular matrix remodeling enzymes; tumor microenvironment.
© 2020 The Authors. Evolutionary Applications published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

