Population genomic data in spider mites point to a role for local adaptation in shaping range shifts
- PMID: 33294025
- PMCID: PMC7691463
- DOI: 10.1111/eva.13086
Population genomic data in spider mites point to a role for local adaptation in shaping range shifts
Abstract
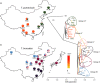
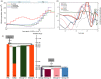
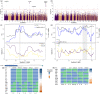
Local adaptation is particularly likely in invertebrate pests that typically have short generation times and large population sizes, but there are few studies on pest species investigating local adaptation and separating this process from contemporaneous and historical gene flow. Here, we use a population genomic approach to investigate evolutionary processes in the two most dominant spider mites in China, Tetranychus truncatus Ehara and Tetranychus pueraricola Ehara et Gotoh, which have wide distributions, short generation times, and large population sizes. We generated genome resequencing of 246 spider mites mostly from China, as well as Japan and Canada at a combined total depth of 3,133×. Based on demographic reconstruction, we found that both mite species likely originated from refugia in southwestern China and then spread to other regions, with the dominant T. truncatus spreading ~3,000 years later than T. pueraricola. Estimated changes in population sizes of the pests matched known periods of glaciation and reinforce the recent expansion of the dominant spider mites. T. truncatus showed a greater extent of local adaptation with more genes (76 vs. 17) associated with precipitation, including candidates involved in regulation of homeostasis of water and ions, signal transduction, and motor skills. In both species, many genes (135 in T. truncatus and 95 in T. pueraricola) also showed signatures of selection related to elevation, including G-protein-coupled receptors, cytochrome P450s, and ABC-transporters. Our results point to historical expansion processes and climatic adaptation in these pests which could have contributed to their growing importance, particularly in the case of T. truncatus.
Keywords: invertebrate pests; local adaptation; range shifts; spider mites; whole‐genome resequencing.
© 2020 The Authors. Evolutionary Applications published by John Wiley & Sons Ltd.
Conflict of interest statement
None declared.
Figures

References
-
- Benton, T. G. , Vickery, J. A. , & Wilson, J. D. (2003). Farmland biodiversity: Is habitat heterogeneity the key? Trends in Ecology & Evolution, 18(4), 182–188. 10.1016/S0169-5347(03)00011-9 - DOI
LinkOut - more resources
Full Text Sources

