A landscape analysis of HIV cure-related clinical research in 2019
- PMID: 33294212
- PMCID: PMC7695817
- DOI: 10.1016/j.jve.2020.100010
A landscape analysis of HIV cure-related clinical research in 2019
Abstract
Objectives: In 2018, we surveyed investigators conducting HIV cure-related clinical research, drawing on information from the online listing established by Treatment Action Group (TAG). The purpose of the survey was to facilitate a landscape analysis of the field. In 2019, we fielded a second survey in order to provide updated information and assess any shifts in the landscape.
Methods: Trials and observational studies listed as of August 16, 2019 formed the sample set. Survey questions addressed funding, trial development, recruitment, enrollment, participant demographics, antiretroviral therapy status, HIV reservoir assays, invasive procedures, study completion, data sharing and dissemination plans. A survey was sent to the contact(s) for each study. Supplemental information was collected from clinicaltrials.gov and available presentations/publications of study results.
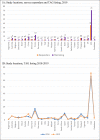
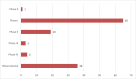
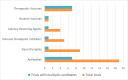
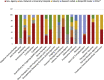
Results: A total of 97 interventional trials and 36 observational studies were identified, with 30 including analytical treatment interruptions. Total projected enrollment is 13,732 participants, with observational studies contributing the majority (8,325). Most interventional trials are in early phases. The majority of current research is located in the USA, involves predominately male participants and is limited in racial and ethnic diversity. Prespecified demographic enrollment targets are rare. Two thirds of respondents to our previous survey reported that enrollment is progressing more slowly than anticipated.
Conclusions: A diverse range of interventions are being evaluated in HIV cure research, but participant diversity is far from optimal with a continuing underrepresentation of women. Broadening inclusion and geographic reach will be necessary to achieve the goal of developing widely effective, safe and accessible curative interventions.
Keywords: Analytical treatment interruptions; Clinical trials registries; HIV cure research; Participant diversity.
© 2020 The Authors.
Conflict of interest statement
All authors declare that they have no competing interests.
Figures
Similar articles
-
A landscape analysis of HIV cure-related clinical trials and observational studies in 2018.J Virus Erad. 2019 Nov 4;5(4):212-219. doi: 10.1016/S2055-6640(20)30030-3. J Virus Erad. 2019. PMID: 31754444 Free PMC article.
-
The future of Cochrane Neonatal.Early Hum Dev. 2020 Nov;150:105191. doi: 10.1016/j.earlhumdev.2020.105191. Epub 2020 Sep 12. Early Hum Dev. 2020. PMID: 33036834
-
Safety and Efficacy of Imatinib for Hospitalized Adults with COVID-19: A structured summary of a study protocol for a randomised controlled trial.Trials. 2020 Oct 28;21(1):897. doi: 10.1186/s13063-020-04819-9. Trials. 2020. PMID: 33115543 Free PMC article.
-
Racial and Ethnic Disparities in Primary Open-Angle Glaucoma Clinical Trials: A Systematic Review and Meta-analysis.JAMA Netw Open. 2021 May 3;4(5):e218348. doi: 10.1001/jamanetworkopen.2021.8348. JAMA Netw Open. 2021. PMID: 34003274 Free PMC article.
-
HIV/AIDS: a minority health issue.Med Clin North Am. 2005 Jul;89(4):895-912. doi: 10.1016/j.mcna.2005.03.005. Med Clin North Am. 2005. PMID: 15925655 Review.
Cited by
-
Participant experiences in HIV cure-directed trial with an extended analytical treatment interruption in Philadelphia, United States.HIV Res Clin Pract. 2023 Oct 6;24(1):2267825. Epub 2023 Oct 14. HIV Res Clin Pract. 2023. PMID: 37837376 Free PMC article.
-
'It is scary to pause treatment': perspectives on HIV cure-related research and analytical treatment interruptions from women diagnosed during acute HIV in Durban, South Africa.HIV Res Clin Pract. 2025 Dec;26(1):2455917. doi: 10.1080/25787489.2025.2455917. Epub 2025 Jan 25. HIV Res Clin Pract. 2025. PMID: 39862155
-
Preliminary Acceptability of a Home-Based Peripheral Blood Collection Device for Viral Load Testing in the Context of Analytical Treatment Interruptions in HIV Cure Trials: Results from a Nationwide Survey in the United States.J Pers Med. 2022 Feb 7;12(2):231. doi: 10.3390/jpm12020231. J Pers Med. 2022. PMID: 35207719 Free PMC article.
-
Centring the health of women across the HIV research continuum.Lancet HIV. 2024 Mar;11(3):e186-e194. doi: 10.1016/S2352-3018(24)00004-3. Lancet HIV. 2024. PMID: 38417977 Free PMC article. Review.
-
Community HIV clinicians' perceptions about HIV cure-related research in the Northwestern United States.HIV Res Clin Pract. 2022 Jul 18;23(1):61-75. HIV Res Clin Pract. 2022. PMID: 35904107 Free PMC article.
References
-
- Jensen B, Knops E, Lübke N et al. Analytic treatment interruption (ATI) after allogeneic CCR5-D32 HSCT for AML in 2013. Conference on Retroviruses and Opportunistic Infections. March 2019. Seattle, WA, USA. Abstract 394.
-
- Nijhuis M. 10th IAS Conference on HIV Science (IAS2019) July 2019. HIV cure by stem cell transplantation. Mexico City, Mexico. Abstract MOSY0706.
-
- Treatment Action Group Research toward a cure trials. http://www.treatmentactiongroup.org/cure/trials Available at: (accessed December 2019)
LinkOut - more resources
Full Text Sources
Other Literature Sources