ERα is a target for butein-induced growth suppression in breast cancer
- PMID: 33294263
- PMCID: PMC7716169
ERα is a target for butein-induced growth suppression in breast cancer
Abstract
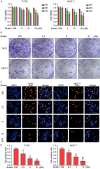
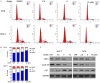
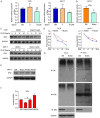
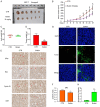
Breast cancer (BCa) has the highest incidence and mortality among malignant diseases in female worldwide. BCa is frequently caused by estrogen receptor α (ERα), a ligand-dependent receptor that highly expressed in about 70% of breast tumors. Therefore, ERα has become a well-characterized and the most effective target for treating ERα-expressing BCa (ERα+ BCa). However, the acquire resistance was somehow developed in patients who received current ERα signaling-targeted endocrine therapies. Hence, discovery of novel anti-estrogen/ERα strategies is urgent. In the present study, we identified butein as a potential agent for breast cancer treatment by the use of a natural product library. We showed that butein inhibits the growth of ERα+ BCa both in vitro and in vivo which is associated with cell cycle arrest that partially triggered by butein-induced ERα downregulation. Mechanically, butein binds to a specific pocket of ERα and promotes proteasome-mediated degradation of the receptor. Collectively, this work reveals that butein is a candidate to diminish ERα signaling which represents a potentially novel strategy for treating BCa.
Keywords: Breast cancer; butein; degradation; estrogen receptor α; growth.
AJCR Copyright © 2020.
Conflict of interest statement
None.
Figures



References
-
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. - PubMed
-
- Reis-Filho JS, Pusztai L. Gene expression profiling in breast cancer: classification, prognostication, and prediction. Lancet. 2011;378:1812–1823. - PubMed
-
- Rouzier R, Perou CM, Symmans WF, Ibrahim N, Cristofanilli M, Anderson K, Hess KR, Stec J, Ayers M, Wagner P, Morandi P, Fan C, Rabiul I, Ross JS, Hortobagyi GN, Pusztai L. Breast cancer molecular subtypes respond differently to preoperative chemotherapy. Clin Cancer Res. 2005;11:5678–5685. - PubMed
-
- Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA, Fluge O, Pergamenschikov A, Williams C, Zhu SX, Lonning PE, Borresen-Dale AL, Brown PO, Botstein D. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. - PubMed
-
- Thomas C, Gustafsson JA. The different roles of ER subtypes in cancer biology and therapy. Nat Rev Cancer. 2011;11:597–608. - PubMed
LinkOut - more resources
Full Text Sources
