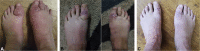
Resolution of pretibial myxedema with teprotumumab in a patient with Graves disease
- PMID: 33294564
- PMCID: PMC7701010
- DOI: 10.1016/j.jdcr.2020.09.003
Resolution of pretibial myxedema with teprotumumab in a patient with Graves disease
Figures
References
-
- Fatourechi V. Pretibial myxedema. Am J Clin Dermatol. 2005;6(5):295–309. - PubMed
-
- Heymann W.R. Teprotumumab: an eye-opening advance for pretibial myxedema? Dermatology World Insights and Inquiries. 2017 https://www.aad.org/dw/dw-insights-and-inquiries/medical-dermatology/tep... Accessed October 24, 2020. Available at:
-
- Ghazi E.R., Heymann W.R. Pretibial myxedema. In: Lebwohl M.G., Heymann W.R., Berth-Jones J., Coulson I., editors. Treatment of Skin Disease. Elsevier; London, Chapter 202: 2018. pp. 668–671.
-
- Alia E., Feit E.J., Levitt J.O. Electrosurgical debulking of pretibial myxedema of the foot. Dermatol Online J. 2019;25(2) 13030/qt4hb7q330. - PubMed
-
- Schwartz K.M., Fatourechi V., Ahmed D.D., Pond G.R. Dermopathy of Graves' disease (pretibial myxedema): long-term outcome. J Clin Endocrinol Metab. 2002;87(2):438–446. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
