In vivo exposure to codeine induces reproductive toxicity: role of HER2 and p53/Bcl-2 signaling pathway
- PMID: 33294712
- PMCID: PMC7695972
- DOI: 10.1016/j.heliyon.2020.e05589
In vivo exposure to codeine induces reproductive toxicity: role of HER2 and p53/Bcl-2 signaling pathway
Abstract
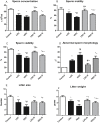
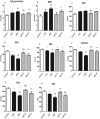
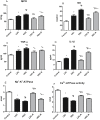
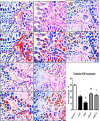
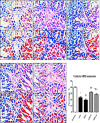
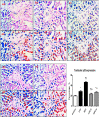
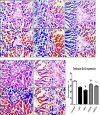
Several studies have implicated codeine use in the aetiopathogenesis of male infertility. The purpose of this study was to investigate the role of HER2, Ki67, oestrogen and p53/Bcl-2 signaling pathways and the possible outcome of codeine cessation on codeine-induced reproductive toxicity. Thirty adult male Wistar rats of comparable ages and weights were randomly allocated into 5 groups. The control animals received distilled water per os (p.o), while animals in the low-dose (LDC) and high dose (HDC) codeine-treated groups received 2 and 5 mg/kg/day of codeine respectively p.o for 6 weeks. The animals in the low-dose codeine recovery (LDC-R) and high-dose codeine recovery (HDC-R) groups received treatment as LDC and HDC respectively followed by another drug-free six weeks, recovery period. Cessation of codeine exposure led to a partial reversal of codeine-induced poor sperm quality, reduced litter size and weight, increased oxidative testicular injury, testicular apoptosis, and testicular DNA damage caused by codeine administration. Codeine-induced gonado-spermotoxicity was associated with a reduction of circulatory testosterone, suppression of testicular HER2, Ki67, and Bcl-2 expression, down-regulation of oestrogen signaling, and upregulation of testicular caspase 3 activities and p53 signaling pathway. Conclusion: Upregulation of oestrogen signaling associated with enhanced testicular HER2 and Ki67 expression during the recovery period is seemingly beneficial in protecting against codeine-related testicular injury and infertility.
Keywords: Bcl-2; Biological sciences; Chemistry; Environmental science; HER2; Health sciences; Ki67; Opioid; Oxidative stress; Veterinary medicine; p53.
© 2020 The Author(s).
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Testicular toxicity following chronic codeine administration is via oxidative DNA damage and up-regulation of NO/TNF-α and caspase 3 activities.PLoS One. 2020 Mar 13;15(3):e0224052. doi: 10.1371/journal.pone.0224052. eCollection 2020. PLoS One. 2020. PMID: 32168344 Free PMC article.
-
In vivo exposure to bisphenol F induces oxidative testicular toxicity: role of Erβ and p53/Bcl-2 signaling pathway.Front Reprod Health. 2023 Aug 2;5:1204728. doi: 10.3389/frph.2023.1204728. eCollection 2023. Front Reprod Health. 2023. PMID: 37601897 Free PMC article.
-
NTP technical report on the toxicity studies of Dibutyl Phthalate (CAS No. 84-74-2) Administered in Feed to F344/N Rats and B6C3F1 Mice.Toxic Rep Ser. 1995 Apr;30:1-G5. Toxic Rep Ser. 1995. PMID: 12209194
-
Thymoquinone attenuates testicular and spermotoxicity following subchronic lead exposure in male rats: Possible mechanisms are involved.Life Sci. 2019 Aug 1;230:132-140. doi: 10.1016/j.lfs.2019.05.067. Epub 2019 May 25. Life Sci. 2019. PMID: 31136753
-
NTP Toxicology and Carcinogenesis Studies of Codeine (CAS No. 76-57-3) in F344 Rats and B6C3F1 Mice (Feed Studies).Natl Toxicol Program Tech Rep Ser. 1996 Aug;455:1-275. Natl Toxicol Program Tech Rep Ser. 1996. PMID: 12587021
Cited by
-
Influence of ejaculatory abstinence period on semen quality of 5165 normozoospermic and oligozoospermic Nigerian men: A retrospective study.Health Sci Rep. 2022 Aug 25;5(5):e722. doi: 10.1002/hsr2.722. eCollection 2022 Sep. Health Sci Rep. 2022. PMID: 36032514 Free PMC article.
-
Determinant genetic markers of semen quality in livestock.Front Endocrinol (Lausanne). 2024 Oct 4;15:1456305. doi: 10.3389/fendo.2024.1456305. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 39429738 Free PMC article. Review.
-
Restoration of Hepatic and Intestinal Integrity by Phyllanthus amarus Is Dependent on Bax/Caspase 3 Modulation in Intestinal Ischemia-/Reperfusion-Induced Injury.Molecules. 2022 Aug 9;27(16):5073. doi: 10.3390/molecules27165073. Molecules. 2022. PMID: 36014309 Free PMC article.
-
L-Arginine reverses maternal and pre-pubertal codeine exposure-induced sexual dysfunction via upregulation of androgen receptor gene and NO/cGMP signaling.PLoS One. 2022 Sep 13;17(9):e0274411. doi: 10.1371/journal.pone.0274411. eCollection 2022. PLoS One. 2022. PMID: 36099318 Free PMC article.
-
Effects of Titanium Dioxide Nanoparticles on Porcine Prepubertal Sertoli Cells: An "In Vitro" Study.Front Endocrinol (Lausanne). 2022 Jan 3;12:751915. doi: 10.3389/fendo.2021.751915. eCollection 2021. Front Endocrinol (Lausanne). 2022. PMID: 35046890 Free PMC article.
References
-
- Cummins T., Miller S. The effects of drug abuse on sexual functioning. In: Levine S.B., editor. Handbook of Clinical Sexuality for Mental Health Professionals. Brunner-Routledge; New York: 2003. pp. 443–456.
-
- INCB, International Narcotics Control Board. Narcotic Drugs Estimated World Requirements for 2012. Vienna.
-
- Ajayi A.F., Akhigbe R.E. Assessment of sexual behaviour and fertility indices in male rabbits following chronic codeine use. Andrology. 2020;8:509–515. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

