Bioinspired mineralized collagen scaffolds for bone tissue engineering
- PMID: 33294729
- PMCID: PMC7680706
- DOI: 10.1016/j.bioactmat.2020.11.004
Bioinspired mineralized collagen scaffolds for bone tissue engineering
Abstract
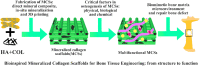
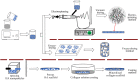
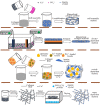
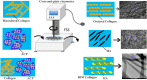
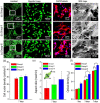
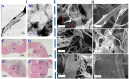
Successful regeneration of large segmental bone defects remains a major challenge in clinical orthopedics, thus it is of important significance to fabricate a suitable alternative material to stimulate bone regeneration. Due to their excellent biocompatibility, sufficient mechanical strength, and similar structure and composition of natural bone, the mineralized collagen scaffolds (MCSs) have been increasingly used as bone substitutes via tissue engineering approaches. Herein, we thoroughly summarize the state of the art of MCSs as tissue-engineered scaffolds for acceleration of bone repair, including their fabrication methods, critical factors for osteogenesis regulation, current opportunities and challenges in the future. First, the current fabrication methods for MCSs, mainly including direct mineral composite, in-situ mineralization and 3D printing techniques, have been proposed to improve their biomimetic physical structures in this review. Meanwhile, three aspects of physical (mechanics and morphology), biological (cells and growth factors) and chemical (composition and cross-linking) cues are described as the critical factors for regulating the osteogenic feature of MCSs. Finally, the opportunities and challenges associated with MCSs as bone tissue-engineered scaffolds are also discussed to point out the future directions for building the next generation of MCSs that should be endowed with satisfactorily mimetic structures and appropriately biological characters for bone regeneration.
Keywords: 3D printing; Biomechanics; Bone repair; Collagen; Mineralization; Scaffold.
© 2020 [The Author/The Authors].
Conflict of interest statement
None.
Figures





References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

