Osteopontin Links Myeloid Activation and Disease Progression in Systemic Sclerosis
- PMID: 33294861
- PMCID: PMC7691442
- DOI: 10.1016/j.xcrm.2020.100140
Osteopontin Links Myeloid Activation and Disease Progression in Systemic Sclerosis
Abstract
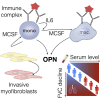
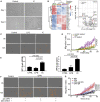
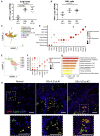
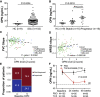
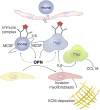
Progressive lung fibrosis is a major cause of mortality in systemic sclerosis (SSc) patients, but the underlying mechanisms remain unclear. We demonstrate that immune complexes (ICs) activate human monocytes to promote lung fibroblast migration partly via osteopontin (OPN) secretion, which is amplified by autocrine monocyte colony stimulating factor (MCSF) and interleukin-6 (IL-6) activity. Bulk and single-cell RNA sequencing demonstrate that elevated OPN expression in SSc lung tissue is enriched in macrophages, partially overlapping with CCL18 expression. Serum OPN is elevated in SSc patients with interstitial lung disease (ILD) and prognosticates future lung function deterioration in SSc cohorts. Serum OPN levels decrease following tocilizumab (monoclonal anti-IL-6 receptor) treatment, confirming the connection between IL-6 and OPN in SSc patients. Collectively, these data suggest a plausible link between autoantibodies and lung fibrosis progression, where circulating OPN serves as a systemic proxy for IC-driven profibrotic macrophage activity, highlighting its potential as a promising biomarker in SSc ILD.
Trial registration: ClinicalTrials.gov NCT01532869 NCT02453256.
Keywords: IL-6; ILD; SPP1; SSc; biomarker; fibrosis; immune complex; macrophages; osteopontin; systemic sclerosis.
© 2020 The Author(s).
Conflict of interest statement
All authors except P.J.W. and D.K. are current or past employees of Genentech, a member of the Roche group, and may hold Roche stock or stock options. D.K. is a consultant to Actelion, Acceleron, Arena, Bayer, Boehringer Ingelheim, Bristol-Myer Squibb, CSL Behring, Corbus, Cytori, GSK, Genentech/ Roche, Galapagos, Merck, Mitsubishi Tanabi, and UCB. He has received grants as part of investigator-initiated trials (to the University of Michigan) from Bayer, Bristol-Myer Squibb, and Pfizer and has stock options in Eicos Sciences, Inc. A.J. is a former employee of Genentech and is now employed by Gilead Sciences (Foster City, CA, USA).
Figures

References
-
- Denton C.P., Khanna D. Systemic sclerosis. Lancet. 2017;390:1685–1699. - PubMed
-
- Furst D., Pope J., Seibold J., Bombardieri S., Denton C., Distler O., Kahaleh B., Kennedy A., Khanna D., Lafyatis R. Progress and priorities in systemic sclerosis: the next 10 years – report from the World Scleroderma Foundation. JSRD. 2016;1:7–9.
-
- Nihtyanova S.I., Denton C.P. Autoantibodies as predictive tools in systemic sclerosis. Nat. Rev. Rheumatol. 2010;6:112–116. - PubMed
-
- Walker U.A., Tyndall A., Czirják L., Denton C., Farge-Bancel D., Kowal-Bielecka O., Müller-Ladner U., Bocelli-Tyndall C., Matucci-Cerinic M. Clinical risk assessment of organ manifestations in systemic sclerosis: a report from the EULAR Scleroderma Trials And Research group database. Ann. Rheum. Dis. 2007;66:754–763. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

