The Potential for Repurposing Anti-TNF as a Therapy for the Treatment of COVID-19
- PMID: 33294881
- PMCID: PMC7713589
- DOI: 10.1016/j.medj.2020.11.005
The Potential for Repurposing Anti-TNF as a Therapy for the Treatment of COVID-19
Abstract
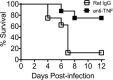
Coronavirus disease 2019 (COVID-19) currently has few effective treatments. Given the uncertainty surrounding the effectiveness and uptake of a vaccine, it is important that the search for treatments continue. An exaggerated inflammatory state is likely responsible for much of the morbidity and mortality in COVID-19. Elevated levels of tumor necrosis factor (TNF), a key pro-inflammatory cytokine, have been shown to be associated with increased COVID-19 mortality. In patients with rheumatoid arthritis, TNF blockade reduces not only biologically active TNF but other pro-inflammatory cytokines important in COVID-19 hyperinflammation. Observational data from patients already on anti-TNF therapy show a reduced rate of COVID-19 poor outcomes and death compared with other immune-suppressing therapies. Anti-TNF has a long history of safe use, including in special at-risk populations, and is widely available. The case to adequately assess anti-TNF as a treatment for COVID-19 is compelling.
Keywords: SARS-CoV-2; coronavirus disease-2019; glucocorticoids; pandemic; tumor necrosis factor.
Crown Copyright © 2020 Published by Elsevier Inc.
Conflict of interest statement
P.C.R. has received personal fees from AbbVie, Eli Lilly, Gilead, Janssen, Novartis, Pfizer, Roche, and UCB; research grant funding from UCB, Janssen, Pfizer, and Novartis; and non-financial support from Bristol Myers Squibb (all unrelated to this work). D.R. has received personal fees for consultancy on drug safety from GlaxoSmithKline unrelated to the topic of this work. M.F. has held patents, now expired, on the use of infliximab and methotrexate in inflammatory arthritis and has received royalties (now ceased) from Johnson & Johnson, AbbVie, Amgen, and UCB, unrelated to this work.
Figures
Similar articles
-
Evaluation of the frequency and intensity of COVID-19 in patients with ankylosing spondylitis under anti-TNF therapy.Turk J Med Sci. 2022 Apr;52(2):522-523. doi: 10.55730/1300-0144.5341. Epub 2022 Apr 14. Turk J Med Sci. 2022. PMID: 36161620 Free PMC article.
-
Therapeutic use of specific tumour necrosis factor inhibitors in inflammatory diseases including COVID-19.Biomed Pharmacother. 2021 Aug;140:111785. doi: 10.1016/j.biopha.2021.111785. Epub 2021 May 28. Biomed Pharmacother. 2021. PMID: 34126316 Free PMC article. Review.
-
Clinical outcomes of patients with COVID-19 and inflammatory rheumatic diseases receiving biological/targeted therapy.Ann Saudi Med. 2022 May-Jun;42(3):155-164. doi: 10.5144/0256-4947.2022.155. Epub 2022 Jun 2. Ann Saudi Med. 2022. PMID: 35658585 Free PMC article.
-
Blocking TNF signaling may save lives in COVID-19 infection.Mol Biol Rep. 2022 Mar;49(3):2303-2309. doi: 10.1007/s11033-022-07166-x. Epub 2022 Jan 25. Mol Biol Rep. 2022. PMID: 35076845 Free PMC article. Review.
-
Prospective Roles of Tumor Necrosis Factor-Alpha (TNF-α) in COVID-19: Prognosis, Therapeutic and Management.Int J Mol Sci. 2023 Mar 24;24(7):6142. doi: 10.3390/ijms24076142. Int J Mol Sci. 2023. PMID: 37047115 Free PMC article. Review.
Cited by
-
Immunosuppressant Therapies in COVID-19: Is the TNF Axis an Alternative?Pharmaceuticals (Basel). 2022 May 17;15(5):616. doi: 10.3390/ph15050616. Pharmaceuticals (Basel). 2022. PMID: 35631442 Free PMC article. Review.
-
The Role of the PFNA Operon of Bifidobacteria in the Recognition of Host's Immune Signals: Prospects for the Use of the FN3 Protein in the Treatment of COVID-19.Int J Mol Sci. 2021 Aug 26;22(17):9219. doi: 10.3390/ijms22179219. Int J Mol Sci. 2021. PMID: 34502130 Free PMC article. Review.
-
Role of CCL2/CCR2 axis in the pathogenesis of COVID-19 and possible Treatments: All options on the Table.Int Immunopharmacol. 2022 Dec;113(Pt A):109325. doi: 10.1016/j.intimp.2022.109325. Epub 2022 Oct 14. Int Immunopharmacol. 2022. PMID: 36252475 Free PMC article. Review.
-
Anti-TNFα Drugs and Interleukin Inhibitors: Epidemiological and Pharmacovigilance Investigation in COVID-19 Positive Patients.J Pers Med. 2022 Oct 27;12(11):1770. doi: 10.3390/jpm12111770. J Pers Med. 2022. PMID: 36579506 Free PMC article.
-
Potential Pathophysiological Mechanisms Underlying Multiple Organ Dysfunction in Cytokine Release Syndrome.Mediators Inflamm. 2022 Apr 6;2022:7137900. doi: 10.1155/2022/7137900. eCollection 2022. Mediators Inflamm. 2022. PMID: 35431655 Free PMC article. Review.
References
-
- Amigues I., Pearlman A.H., Patel A., Reid P., Robinson P.C., Sinha R., Kim A.H., Youngstein T., Jayatilleke A., Konig M.F. Coronavirus disease 2019: investigational therapies in the prevention and treatment of hyperinflammation. Expert Rev. Clin. Immunol. 2020 doi: 10.1080/1744666X.2021.1847084. Published online November 5, 2020. - DOI - PMC - PubMed
-
- Stone J.H., Frigault M.J., Serling-Boyd N.J., Fernandes A.D., Harvey L., Foulkes A.S., Horick N.K., Healy B.C., Shah R., Bensaci A.M., BACC Bay Tocilizumab Trial Investigators Efficacy of tocilizumab in patients hospitalized with COVID-19. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2028836. Published online October 21, 2020. - DOI - PMC - PubMed
-
- Hermine O., Mariette X., Tharaux P.-L., Resche-Rigon M., Porcher R., Ravaud P., CORIMUNO-19 Collaborative Group Effect of tocilizumab vs usual care in adults hospitalized with COVID-19 and moderate or severe pneumonia: a randomized clinical trial. JAMA Intern. Med. 2020 doi: 10.1001/jamainternmed.2020.6820. Published online October 20, 2020. - DOI - PMC - PubMed
-
- Salvarani C., Dolci G., Massari M., Merlo D.F., Cavuto S., Savoldi L., Bruzzi P., Boni F., Braglia L., Turrà C., RCT-TCZ-COVID-19 Study Group Effect of tocilizumab vs standard care on clinical worsening in patients hospitalized with COVID-19 pneumonia: a randomized clinical trial. JAMA Intern. Med. 2020 doi: 10.1001/jamainternmed.2020.6615. Published online October 20, 2020. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous