The Potential for Repurposing Anti-TNF as a Therapy for the Treatment of COVID-19
- PMID: 33294881
- PMCID: PMC7713589
- DOI: 10.1016/j.medj.2020.11.005
The Potential for Repurposing Anti-TNF as a Therapy for the Treatment of COVID-19
Abstract
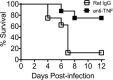
Coronavirus disease 2019 (COVID-19) currently has few effective treatments. Given the uncertainty surrounding the effectiveness and uptake of a vaccine, it is important that the search for treatments continue. An exaggerated inflammatory state is likely responsible for much of the morbidity and mortality in COVID-19. Elevated levels of tumor necrosis factor (TNF), a key pro-inflammatory cytokine, have been shown to be associated with increased COVID-19 mortality. In patients with rheumatoid arthritis, TNF blockade reduces not only biologically active TNF but other pro-inflammatory cytokines important in COVID-19 hyperinflammation. Observational data from patients already on anti-TNF therapy show a reduced rate of COVID-19 poor outcomes and death compared with other immune-suppressing therapies. Anti-TNF has a long history of safe use, including in special at-risk populations, and is widely available. The case to adequately assess anti-TNF as a treatment for COVID-19 is compelling.
Keywords: SARS-CoV-2; coronavirus disease-2019; glucocorticoids; pandemic; tumor necrosis factor.
Crown Copyright © 2020 Published by Elsevier Inc.
Conflict of interest statement
P.C.R. has received personal fees from AbbVie, Eli Lilly, Gilead, Janssen, Novartis, Pfizer, Roche, and UCB; research grant funding from UCB, Janssen, Pfizer, and Novartis; and non-financial support from Bristol Myers Squibb (all unrelated to this work). D.R. has received personal fees for consultancy on drug safety from GlaxoSmithKline unrelated to the topic of this work. M.F. has held patents, now expired, on the use of infliximab and methotrexate in inflammatory arthritis and has received royalties (now ceased) from Johnson & Johnson, AbbVie, Amgen, and UCB, unrelated to this work.
Figures
References
-
- Amigues I., Pearlman A.H., Patel A., Reid P., Robinson P.C., Sinha R., Kim A.H., Youngstein T., Jayatilleke A., Konig M.F. Coronavirus disease 2019: investigational therapies in the prevention and treatment of hyperinflammation. Expert Rev. Clin. Immunol. 2020 doi: 10.1080/1744666X.2021.1847084. Published online November 5, 2020. - DOI - PMC - PubMed
-
- Stone J.H., Frigault M.J., Serling-Boyd N.J., Fernandes A.D., Harvey L., Foulkes A.S., Horick N.K., Healy B.C., Shah R., Bensaci A.M., BACC Bay Tocilizumab Trial Investigators Efficacy of tocilizumab in patients hospitalized with COVID-19. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2028836. Published online October 21, 2020. - DOI - PMC - PubMed
-
- Hermine O., Mariette X., Tharaux P.-L., Resche-Rigon M., Porcher R., Ravaud P., CORIMUNO-19 Collaborative Group Effect of tocilizumab vs usual care in adults hospitalized with COVID-19 and moderate or severe pneumonia: a randomized clinical trial. JAMA Intern. Med. 2020 doi: 10.1001/jamainternmed.2020.6820. Published online October 20, 2020. - DOI - PMC - PubMed
-
- Salvarani C., Dolci G., Massari M., Merlo D.F., Cavuto S., Savoldi L., Bruzzi P., Boni F., Braglia L., Turrà C., RCT-TCZ-COVID-19 Study Group Effect of tocilizumab vs standard care on clinical worsening in patients hospitalized with COVID-19 pneumonia: a randomized clinical trial. JAMA Intern. Med. 2020 doi: 10.1001/jamainternmed.2020.6615. Published online October 20, 2020. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous