Ubiquitously specific protease 4 inhibitor-Vialinin A attenuates inflammation and fibrosis in S100-induced hepatitis mice through Rheb/mTOR signalling
- PMID: 33295107
- PMCID: PMC7812299
- DOI: 10.1111/jcmm.16180
Ubiquitously specific protease 4 inhibitor-Vialinin A attenuates inflammation and fibrosis in S100-induced hepatitis mice through Rheb/mTOR signalling
Abstract
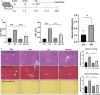
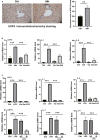
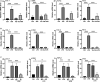
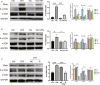
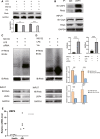
Inflammation and fibrosis are major consequences of autoimmune hepatitis, however, the therapeutic mechanism remains to be investigated. USP4 is a deubiquitinating enzyme and plays an important role in tissue fibrosis and immune disease. Vialinin A is an extract from mushroom and is a specific USP4 inhibitor. However, there is lack of evidences that Vialinin A plays a role in autoimmune hepatitis. By employing S100-induced autoimmune hepatitis in mice and AML12 cell line, therapeutic mechanism of Vialinin A was examined. Inflammation was documented by liver histological staining and inflammatory cytokines. Fibrosis was demonstrated by Masson, Sirius red staining and fibrotic cytokines with western blot and real-time RT-PCR. In experimental animal, there were increases in inflammation and fibrosis as well as USP4, and which were reduced after treatment of Vialinin A. Vialinin A also reduced Rheb and phosphorylated mTOR. Moreover, in LPS-treated AML12 cells, LPS-induced USP4, inflammatory and fibrotic cytokines, phosphorylated mTOR and Rheb. Specific inhibitory siRNA of USP4 reduced USP4 level and the parameters mentioned above. In conclusion, USP4 was significantly elevated in autoimmune hepatitis mice and Vialinin A reduced USP4 level and attenuate inflammation and fibrosis in the liver. The mechanism may be related to regulation of Rheb/mTOR signalling.
Keywords: USP4; Vialinin A; autoimmune hepatitis; mTOR.
© 2020 The Authors. Journal of Cellular and Molecular Medicine published by Foundation for Cellular and Molecular Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
All authors have no potential conflict of interest to declare. All authors read and approved of the final version of the manuscript. All authors have read the journal's policy on conflicts of interest. All authors have read the journal's authorship agreement.
Figures

References
-
- Webb GJ, Hirschfield GM, Krawitt EL, Gershwin ME. Cellular and molecular mechanisms of autoimmune hepatitis. Annu Rev Pathol. 2018;13:247‐292. - PubMed
-
- Lapierre P, Béland K, Alvarez F. Pathogenesis of autoimmune hepatitis: from break of tolerance to immune‐mediated hepatocyte apoptosis. Transl Res. 2007;149(3):107‐113. - PubMed
-
- European Association for the Study of the Liver . EASL clinical practice guidelines: autoimmune hepatitis. J Hepatol. 2015;63(4):971‐1004. - PubMed
-
- Dyson JK, De Martin E, Dalekos GN, et al. Review article: unanswered clinical and research questions in autoimmune hepatitis‐conclusions of the International Autoimmune Hepatitis Group Research Workshop. Aliment Pharmacol Ther. 2019;49(5):528–536. - PubMed
-
- Trivedi PJ, Hubscher SG, Heneghan M, Gleeson D, Hirschfield GM. Grand round: autoimmune hepatitis. J Hepatol. 2019;70(4):773–784. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 81570514/National Natural Science Foundation of China (NSFC)
- 81770585/National Natural Science Foundation of China (NSFC)
- 81600466/National Natural Science Foundation of China (NSFC)
- 2017ZX10202201/National Science and Technology Major Project
- 2017ZX10203201/National Science and Technology Major Project
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

