Mucocutaneous Manifestations of Multisystem Inflammatory Syndrome in Children During the COVID-19 Pandemic
- PMID: 33295957
- PMCID: PMC7726702
- DOI: 10.1001/jamadermatol.2020.4779
Mucocutaneous Manifestations of Multisystem Inflammatory Syndrome in Children During the COVID-19 Pandemic
Abstract
Importance: To date, no study has characterized the mucocutaneous features seen in hospitalized children with multisystem inflammatory syndrome in children (MIS-C) or the temporal association of these findings with the onset of systemic symptoms.
Objective: To describe the mucocutaneous findings seen in children with MIS-C during the height of the coronavirus disease 2019 (COVID-19) pandemic in New York City in 2020.
Design, setting, and participants: A retrospective case series was conducted of 35 children admitted to 2 hospitals in New York City between April 1 and July 14, 2020, who met Centers for Disease Control and Prevention and/or epidemiologic criteria for MIS-C.
Main outcomes and measures: Laboratory and clinical characteristics, with emphasis on mucocutaneous findings, of children who met criteria for MIS-C. The characterization of mucocutaneous features was verified by 2 board-certified pediatric dermatologists.
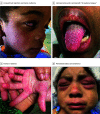
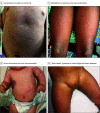
Results: Twenty-five children (11 girls [44%]; median age, 3 years [range, 0.7-17 years]) were identified who met definitional criteria for MIS-C; an additional 10 children (5 girls [50%]; median age, 1.7 years [range, 0.2-15 years]) were included as probable MIS-C cases (patients met all criteria with the exception of laboratory test evidence of severe acute respiratory syndrome coronavirus 2 [SARS-CoV-2] infection or known exposure). The results of polymerase chain reaction tests for SARS-CoV-2 were positive for 10 patients (29%), and the results of SARS-CoV-2 immunoglobulin G tests were positive for 19 patients (54%). Of the 35 patients, 29 (83%) exhibited mucocutaneous changes, with conjunctival injection (n = 21), palmoplantar erythema (n = 18), lip hyperemia (n = 17), periorbital erythema and edema (n = 7), strawberry tongue (n = 8), and malar erythema (n = 6) being the most common findings. Recognition of mucocutaneous findings occurred a mean of 2.7 days (range, 1-7 days) after the onset of fever. The duration of mucocutaneous findings varied from hours to days (median duration, 5 days [range, 0-11 days]). Neither the presence nor absence of mucocutaneous findings was significantly associated with overall disease severity.
Conclusions and relevance: In this case series of hospitalized children with suspected MIS-C during the COVID-19 pandemic, a wide spectrum of mucocutaneous findings was identified. Despite their protean and transient nature, these mucocutaneous features serve as important clues in the recognition of MIS-C.
Conflict of interest statement
Figures
Similar articles
-
Mucocutaneous disease and related clinical characteristics in hospitalized children and adolescents with COVID-19 and multisystem inflammatory syndrome in children.J Am Acad Dermatol. 2021 Feb;84(2):408-414. doi: 10.1016/j.jaad.2020.10.060. Epub 2020 Oct 24. J Am Acad Dermatol. 2021. PMID: 33323343 Free PMC article.
-
Dermatologic manifestations of multisystem inflammatory syndrome in children during the COVID-19 pandemic.An Bras Dermatol. 2023 Mar-Apr;98(2):168-175. doi: 10.1016/j.abd.2022.08.003. Epub 2022 Nov 16. An Bras Dermatol. 2023. PMID: 36473757 Free PMC article.
-
Clinical Characteristics of 58 Children With a Pediatric Inflammatory Multisystem Syndrome Temporally Associated With SARS-CoV-2.JAMA. 2020 Jul 21;324(3):259-269. doi: 10.1001/jama.2020.10369. JAMA. 2020. PMID: 32511692 Free PMC article.
-
Shock and Myocardial Injury in Children With Multisystem Inflammatory Syndrome Associated With SARS-CoV-2 Infection: What We Know. Case Series and Review of the Literature.J Intensive Care Med. 2021 Apr;36(4):392-403. doi: 10.1177/0885066620969350. Epub 2020 Nov 5. J Intensive Care Med. 2021. PMID: 33148089 Review.
-
Oral manifestations of COVID-2019-related multisystem inflammatory syndrome in children: a review of 47 pediatric patients.J Am Dent Assoc. 2021 Mar;152(3):202-208. doi: 10.1016/j.adaj.2020.11.014. Epub 2020 Dec 9. J Am Dent Assoc. 2021. PMID: 33632409 Free PMC article. Review.
Cited by
-
MIS-C related to SARS-CoV-2 infection: a narrative review of presentation, differential diagnosis, and management.Infez Med. 2022 Sep 1;30(3):344-352. doi: 10.53854/liim-3003-3. eCollection 2022. Infez Med. 2022. PMID: 36148163 Free PMC article. Review.
-
Clinical Patterns and Morphology of COVID-19 Dermatology.Dermatol Clin. 2021 Oct;39(4):487-503. doi: 10.1016/j.det.2021.05.006. Epub 2021 May 31. Dermatol Clin. 2021. PMID: 34556240 Free PMC article. Review.
-
Acral peeling as the sole skin manifestation of COVID-19 in children.Pediatr Dermatol. 2021 May;38(3):664-666. doi: 10.1111/pde.14599. Epub 2021 Apr 15. Pediatr Dermatol. 2021. PMID: 33856063 Free PMC article.
-
Postvaccination Multisystem Inflammatory Syndrome in Adult with No Evidence of Prior SARS-CoV-2 Infection.Emerg Infect Dis. 2022 Feb;28(2):411-414. doi: 10.3201/eid2802.211938. Epub 2021 Dec 1. Emerg Infect Dis. 2022. PMID: 34852213 Free PMC article.
-
SARS-CoV-2 Infection and Vaccination Cutaneous Manifestations for the Inpatient Dermatologist.Curr Dermatol Rep. 2022;11(4):252-262. doi: 10.1007/s13671-022-00374-5. Epub 2022 Oct 19. Curr Dermatol Rep. 2022. PMID: 36274753 Free PMC article. Review.
References
-
- Centers for Disease Control and Prevention Multisystem inflammatory syndrome in children (MIS-C) associated with coronavirus disease 2019 (COVID-19). Published May 14, 2020. Accessed July 20, 2020. https://emergency.cdc.gov/han/2020/han00432.asp
-
- Zachariah P, Johnson CL, Halabi KC, et al. ; Columbia Pediatric COVID-19 Management Group . Epidemiology, clinical features, and disease severity in patients with coronavirus disease 2019 (COVID-19) in a children’s hospital in New York City, New York. JAMA Pediatr. 2020;174(10):e202430. doi:10.1001/jamapediatrics.2020.2430 - DOI - PMC - PubMed
MeSH terms
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

