Effect of a Lower vs Higher Positive End-Expiratory Pressure Strategy on Ventilator-Free Days in ICU Patients Without ARDS: A Randomized Clinical Trial
- PMID: 33295981
- PMCID: PMC7726701
- DOI: 10.1001/jama.2020.23517
Effect of a Lower vs Higher Positive End-Expiratory Pressure Strategy on Ventilator-Free Days in ICU Patients Without ARDS: A Randomized Clinical Trial
Abstract
Importance: It is uncertain whether invasive ventilation can use lower positive end-expiratory pressure (PEEP) in critically ill patients without acute respiratory distress syndrome (ARDS).
Objective: To determine whether a lower PEEP strategy is noninferior to a higher PEEP strategy regarding duration of mechanical ventilation at 28 days.
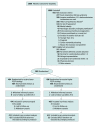
Design, setting, and participants: Noninferiority randomized clinical trial conducted from October 26, 2017, through December 17, 2019, in 8 intensive care units (ICUs) in the Netherlands among 980 patients without ARDS expected not to be extubated within 24 hours after start of ventilation. Final follow-up was conducted in March 2020.
Interventions: Participants were randomized to receive invasive ventilation using either lower PEEP, consisting of the lowest PEEP level between 0 and 5 cm H2O (n = 476), or higher PEEP, consisting of a PEEP level of 8 cm H2O (n = 493).
Main outcomes and measures: The primary outcome was the number of ventilator-free days at day 28, with a noninferiority margin for the difference in ventilator-free days at day 28 of -10%. Secondary outcomes included ICU and hospital lengths of stay; ICU, hospital, and 28- and 90-day mortality; development of ARDS, pneumonia, pneumothorax, severe atelectasis, severe hypoxemia, or need for rescue therapies for hypoxemia; and days with use of vasopressors or sedation.
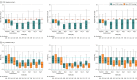
Results: Among 980 patients who were randomized, 969 (99%) completed the trial (median age, 66 [interquartile range {IQR}, 56-74] years; 246 [36%] women). At day 28, 476 patients in the lower PEEP group had a median of 18 ventilator-free days (IQR, 0-27 days) and 493 patients in the higher PEEP group had a median of 17 ventilator-free days (IQR, 0-27 days) (mean ratio, 1.04; 95% CI, 0.95-∞; P = .007 for noninferiority), and the lower boundary of the 95% CI was within the noninferiority margin. Occurrence of severe hypoxemia was 20.6% vs 17.6% (risk ratio, 1.17; 95% CI, 0.90-1.51; P = .99) and need for rescue strategy was 19.7% vs 14.6% (risk ratio, 1.35; 95% CI, 1.02-1.79; adjusted P = .54) in patients in the lower and higher PEEP groups, respectively. Mortality at 28 days was 38.4% vs 42.0% (hazard ratio, 0.89; 95% CI, 0.73-1.09; P = .99) in patients in the lower and higher PEEP groups, respectively. There were no statistically significant differences in other secondary outcomes.
Conclusions and relevance: Among patients in the ICU without ARDS who were expected not to be extubated within 24 hours, a lower PEEP strategy was noninferior to a higher PEEP strategy with regard to the number of ventilator-free days at day 28. These findings support the use of lower PEEP in patients without ARDS.
Trial registration: ClinicalTrials.gov Identifier: NCT03167580.
Conflict of interest statement
Figures


Comment in
-
Searching for the Optimal PEEP in Patients Without ARDS: High, Low, or in Between?JAMA. 2020 Dec 22;324(24):2490-2492. doi: 10.1001/jama.2020.23067. JAMA. 2020. PMID: 33295963 No abstract available.
-
Quantifying the Effect of Lower vs Higher Positive End-Expiratory Pressure on Ventilator-Free Survival in ICU Patients.JAMA. 2021 Apr 20;325(15):1566-1567. doi: 10.1001/jama.2021.1700. JAMA. 2021. PMID: 33877277 No abstract available.
References
-
- Neto AS, Barbas CSV, Simonis FD, et al. ; PRoVENT and PROVE Network Investigators . Epidemiological characteristics, practice of ventilation, and clinical outcome in patients at risk of acute respiratory distress syndrome in intensive care units from 16 countries (PRoVENT): an international, multicentre, prospective study. Lancet Respir Med. 2016;4(11):882-893. doi:10.1016/S2213-2600(16)30305-8 - DOI - PubMed
-
- Serpa Neto A, Filho RR, Cherpanath T, et al. ; PROVE Network Investigators . Associations between positive end-expiratory pressure and outcome of patients without ARDS at onset of ventilation: a systematic review and meta-analysis of randomized controlled trials. Ann Intensive Care. 2016;6(1):109. doi:10.1186/s13613-016-0208-7 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

