Identification and Characterization of Aldehyde Oxidase 5 in the Pheromone Gland of the Silkworm (Lepidoptera: Bombycidae)
- PMID: 33295983
- PMCID: PMC7724976
- DOI: 10.1093/jisesa/ieaa132
Identification and Characterization of Aldehyde Oxidase 5 in the Pheromone Gland of the Silkworm (Lepidoptera: Bombycidae)
Abstract
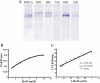
Aldehyde oxidases (AOXs) are a subfamily of cytosolic molybdo-flavoenzymes that play critical roles in the detoxification and degradation of chemicals. Active AOXs, such as AOX1 and AOX2, have been identified and functionally analyzed in insect antennae but are rarely reported in other tissues. This is the first study to isolate and characterize the cDNA that encodes aldehyde oxidase 5 (BmAOX5) in the pheromone gland (PG) of the silkworm, Bombyx mori. The size of BmAOX5 cDNA is 3,741 nucleotides and includes an open reading frame, which encodes a protein of 1,246 amino acid residues. The theoretical molecular weight and isoelectric point of BmAOX5 are approximately 138 kDa and 5.58, respectively. BmAOX5 shares a similar primary structure with BmAOX1 and BmAOX2, containing two [2Fe-2S] redox centers, a FAD-binding domain, and a molybdenum cofactor (MoCo)-binding domain. RT-PCR revealed BmAOX5 to be particularly highly expressed in the PG (including ovipositor) of the female silkworm moth, and the expression was further confirmed by in situ hybridization, AOX activity staining, and anti-BmAOX5 western blotting. Further, BmAOX5 was shown to metabolize aromatic aldehydes, such as benzaldehyde, salicylaldehyde, and vanillic aldehyde, and fatty aldehydes, such as heptaldehyde and propionaldehyde. The maximum reaction rate (Vmax) of benzaldehyde as substrate was 21 mU and Km was 1.745 mmol/liter. These results suggested that BmAOX5 in the PG could metabolize aldehydes in the cytoplasm for detoxification or participate in the degradation of aldehyde pheromone substances and odorant compounds to identify mating partners and locate suitable spawning sites.
Keywords: Bombyx mori; activity staining; aldehyde oxidase; identification; pheromone gland.
© The Author(s) 2020. Published by Oxford University Press on behalf of Entomological Society of America.
Figures



Similar articles
-
Identification and characterization of an antennae-specific aldehyde oxidase from the navel orangeworm.PLoS One. 2013 Jun 24;8(6):e67794. doi: 10.1371/journal.pone.0067794. Print 2013. PLoS One. 2013. PMID: 23826341 Free PMC article.
-
Identification of candidate aldehyde oxidases from the silkworm Bombyx mori potentially involved in antennal pheromone degradation.Gene. 2007 Dec 1;404(1-2):31-40. doi: 10.1016/j.gene.2007.08.022. Epub 2007 Sep 12. Gene. 2007. PMID: 17904312
-
Identification and characterization of aldehyde oxidases (AOXs) in the cotton bollworm.Naturwissenschaften. 2017 Oct 23;104(11-12):94. doi: 10.1007/s00114-017-1515-z. Naturwissenschaften. 2017. PMID: 29063281
-
Evolution, expression, and substrate specificities of aldehyde oxidase enzymes in eukaryotes.J Biol Chem. 2020 Apr 17;295(16):5377-5389. doi: 10.1074/jbc.REV119.007741. Epub 2020 Mar 6. J Biol Chem. 2020. PMID: 32144208 Free PMC article. Review.
-
Structure and function of mammalian aldehyde oxidases.Arch Toxicol. 2016 Apr;90(4):753-80. doi: 10.1007/s00204-016-1683-1. Epub 2016 Feb 26. Arch Toxicol. 2016. PMID: 26920149 Review.
Cited by
-
The Effects of Nine Compounds on Aldehyde-Oxidase-Related Genes in Bactrocera dorsalis (Hendel).Genes (Basel). 2023 Dec 25;15(1):35. doi: 10.3390/genes15010035. Genes (Basel). 2023. PMID: 38254925 Free PMC article.
-
Exploring the Terminal Pathway of Sex Pheromone Biosynthesis and Metabolism in the Silkworm.Insects. 2021 Nov 26;12(12):1062. doi: 10.3390/insects12121062. Insects. 2021. PMID: 34940150 Free PMC article.
-
Characterization of Two Aldehyde Oxidases from the Greater Wax Moth, Galleria mellonella Linnaeus. (Lepidoptera: Pyralidae) with Potential Role as Odorant-Degrading Enzymes.Insects. 2022 Dec 12;13(12):1143. doi: 10.3390/insects13121143. Insects. 2022. PMID: 36555053 Free PMC article.
References
-
- Adachi M., Itoh K., Masubuchi A., Watanabe N., and Tanaka Y.. . 2007. Construction and expression of mutant cDNAs responsible for genetic polymorphism in aldehyde oxidase in Donryu strain rats. J. Biochem. Mol. Biol. 40: 1021–1027. - PubMed
-
- Badwey J. A., Robinson J. M., Karnovsky M. J., and Karnovsky M. L.. . 1981. Superoxide production by an unusual aldehyde oxidase in guinea pig granulocytes. Characterization and cytochemical localization. J. Biol. Chem. 256: 3479–3486. - PubMed
-
- Beedham C., Bruce S. E., Critchley D. J., al-Tayib Y., and Rance D. J.. . 1987. Species variation in hepatic aldehyde oxidase activity. Eur. J. Drug Metab. Pharmacokinet. 12: 307–310. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

