Melflufen and Dexamethasone in Heavily Pretreated Relapsed and Refractory Multiple Myeloma
- PMID: 33296242
- PMCID: PMC8078327
- DOI: 10.1200/JCO.20.02259
Melflufen and Dexamethasone in Heavily Pretreated Relapsed and Refractory Multiple Myeloma
Abstract
Purpose: Melphalan flufenamide (melflufen) is a first-in-class peptide-drug conjugate that targets aminopeptidases and rapidly and selectively releases alkylating agents into tumor cells. The phase II HORIZON trial evaluated the efficacy of melflufen plus dexamethasone in relapsed and refractory multiple myeloma (RRMM), a population with an important unmet medical need.
Patients and methods: Patients with RRMM refractory to pomalidomide and/or an anti-CD38 monoclonal antibody received melflufen 40 mg intravenously on day 1 of each 28-day cycle plus once weekly oral dexamethasone at a dose of 40 mg (20 mg in patients older than 75 years). The primary end point was overall response rate (partial response or better) assessed by the investigator and confirmed by independent review. Secondary end points included duration of response, progression-free survival, overall survival, and safety. The primary analysis is complete with long-term follow-up ongoing.
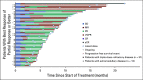
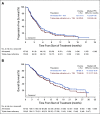
Results: Of 157 patients (median age 65 years; median five prior lines of therapy) enrolled and treated, 119 patients (76%) had triple-class-refractory disease, 55 (35%) had extramedullary disease, and 92 (59%) were refractory to previous alkylator therapy. The overall response rate was 29% in the all-treated population, with 26% in the triple-class-refractory population. In the all-treated population, median duration of response was 5.5 months, median progression-free survival was 4.2 months, and median overall survival was 11.6 months at a median follow-up of 14 months. Grade ≥ 3 treatment-emergent adverse events occurred in 96% of patients, most commonly neutropenia (79%), thrombocytopenia (76%), and anemia (43%). Pneumonia (10%) was the most common grade 3/4 nonhematologic event. Thrombocytopenia and bleeding (both grade 3/4 but fully reversible) occurred concomitantly in four patients. GI events, reported in 97 patients (62%), were predominantly grade 1/2 (93%); none were grade 4.
Conclusion: Melflufen plus dexamethasone showed clinically meaningful efficacy and a manageable safety profile in patients with heavily pretreated RRMM, including those with triple-class-refractory and extramedullary disease.
Trial registration: ClinicalTrials.gov NCT02963493.
Figures
References
-
- Kumar SK Dimopoulos MA Kastritis E, et al. : Natural history of relapsed myeloma, refractory to immunomodulatory drugs and proteasome inhibitors: A multicenter IMWG study. Leukemia 31:2443-2448, 2017 - PubMed
-
- Moreau P San Miguel J Sonneveld P, et al. : Multiple myeloma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 28:iv52-iv61, 2017 - PubMed
-
- Jimenez-Segura R Granell M Gironella M, et al. : Pomalidomide-dexamethasone for treatment of soft-tissue plasmacytomas in patients with relapsed/refractory multiple myeloma. Eur J Haematol 102:389-394, 2019 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

