Clinician-researcher's perspectives on clinical research during the COVID19 pandemic
- PMID: 33296427
- PMCID: PMC7725301
- DOI: 10.1371/journal.pone.0243525
Clinician-researcher's perspectives on clinical research during the COVID19 pandemic
Abstract
Objectives: The outcome of well-performed clinical research is essential for evidence-based patient management during pandemics. However, conducting clinical research amidst a pandemic requires researchers to balance clinical and research demands. We seek to understand the values, experiences, and beliefs of physicians working at the onset of the COVID-19 pandemic in order to inform clinical research planning. We aim to understand whether pandemic settings affect physician comfort with research practices, and how physician experiences shape their understanding of research in a pandemic setting.
Methods: A survey tool was adapted to evaluate familiarity and comfort with research during a pandemic. A cross-sectional, online questionnaire was distributed across Canadian research networks early in the COVID-19 outbreak. The survey was administered between March 11th and 17th, 2020, during a time of local transmission but prior to the surge of cases. We aimed to recruit into the survey physicians in infectious disease and critical care research networks across Canada.
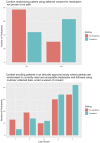
Results: Of the 133 physician respondents, 131 (98%) considered it important to conduct clinical research during the COVID-19 pandemic. Respondents were more accepting of adaptations to the research process in during a pandemic compared to in a non-pandemic setting, including conducting research with deferred consent (χ2 = 8.941, 95% CI: -0.264, -0.085, p = 0.003), using non-identifiable observational data with a waiver of consent with a median score of 97 out of 100 (IQR: 79.25-100) vs median 87 out of 100 (IQR: 63-79) (95% CI: -12.43, 0.054, p = 0.052). The majority felt that research quality is not compromised during pandemics.
Conclusions: Physicians consider it important to conduct research during a pandemic, highlighting the need to expedite research activities in pandemic settings. Respondents were more accepting of adaptations to the research process for research conducted during a pandemic, compared to that conducted in its absence of a pandemic.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- WHO. International clinical trials registry platform: Search portal. Geneva: World Health Organization; [cited 2020 April 14]. https://apps.who.int/trialsearch/Default.aspx.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

