Variable but not random: temporal pattern coding in a songbird brain area necessary for song modification
- PMID: 33296616
- PMCID: PMC7948135
- DOI: 10.1152/jn.00034.2019
Variable but not random: temporal pattern coding in a songbird brain area necessary for song modification
Abstract
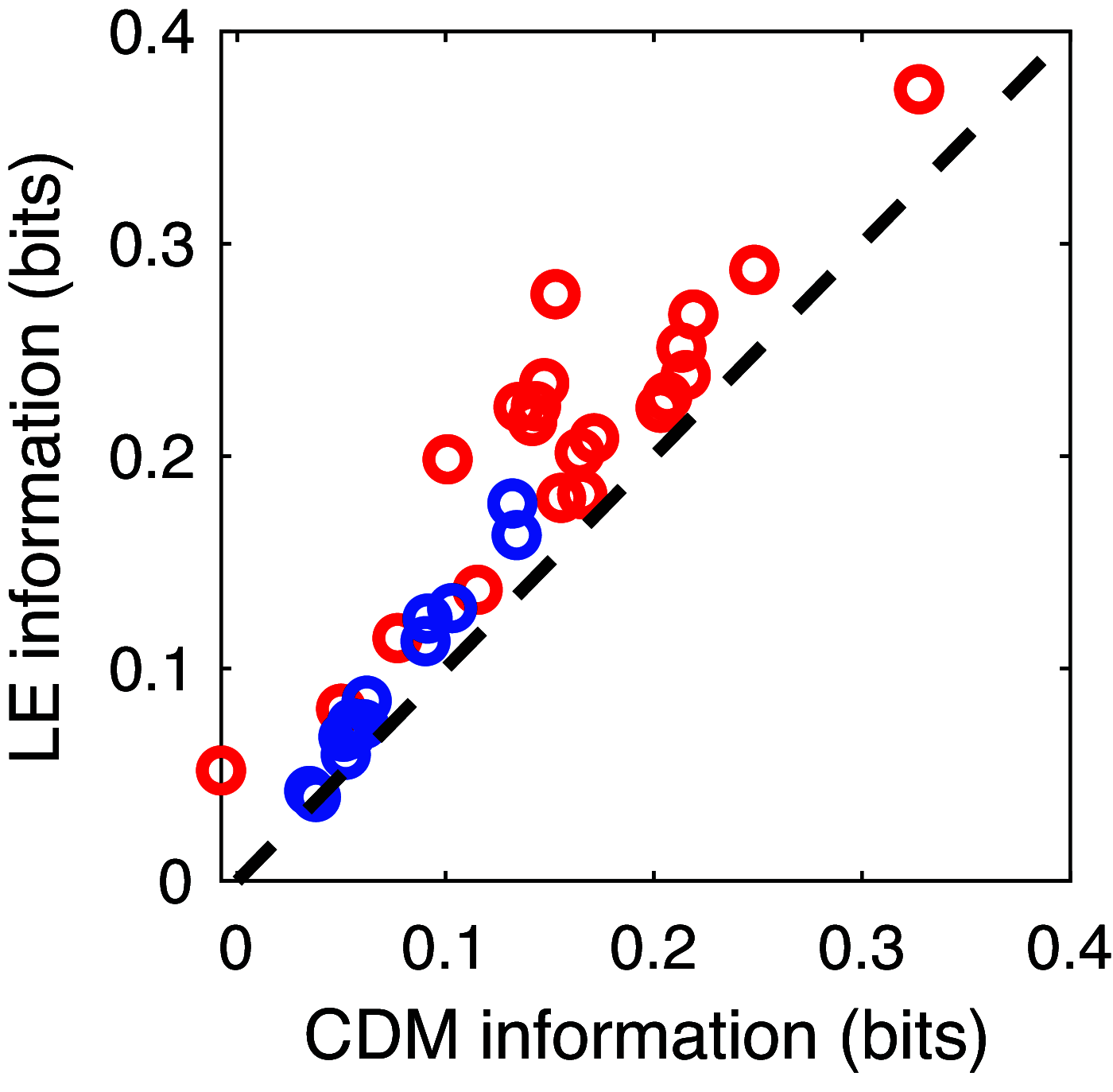
Practice of a complex motor gesture involves motor exploration to attain a better match to target, but little is known about the neural code for such exploration. We examine spiking in a premotor area of the songbird brain critical for song modification and quantify correlations between spiking and time in the motor sequence. While isolated spikes code for time in song during performance of song to a female bird, extended strings of spiking and silence, particularly bursts, code for time in song during undirected (solo) singing, or "practice." Bursts code for particular times in song with more information than individual spikes, and this spike-spike synergy is significantly higher during undirected singing. The observed pattern information cannot be accounted for by a Poisson model with a matched time-varying rate, indicating that the precise timing of spikes in both bursts in undirected singing and isolated spikes in directed singing code for song with a temporal code. Temporal coding during practice supports the hypothesis that lateral magnocellular nucleus of the anterior nidopallium neurons actively guide song modification at local instances in time.NEW & NOTEWORTHY This paper shows that bursts of spikes in the songbird brain during practice carry information about the output motor pattern. The brain's code for song changes with social context, in performance versus practice. Synergistic combinations of spiking and silence code for time in the bird's song. This is one of the first uses of information theory to quantify neural information about a motor output. This activity may guide changes to the song.
Keywords: birdsong; information theory; motor performance; motor practice; temporal coding.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures







References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

