Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes, in Contrast to Adipose Tissue-Derived Stromal Cells, Efficiently Improve Heart Function in Murine Model of Myocardial Infarction
- PMID: 33297443
- PMCID: PMC7762393
- DOI: 10.3390/biomedicines8120578
Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes, in Contrast to Adipose Tissue-Derived Stromal Cells, Efficiently Improve Heart Function in Murine Model of Myocardial Infarction
Abstract
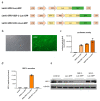
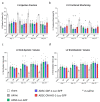
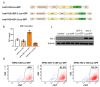
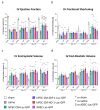
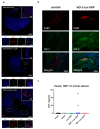
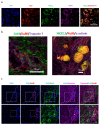
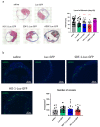
Cell therapies are extensively tested to restore heart function after myocardial infarction (MI). Survival of any cell type after intracardiac administration, however, may be limited due to unfavorable conditions of damaged tissue. Therefore, the aim of this study was to evaluate the therapeutic effect of adipose-derived stromal cells (ADSCs) and human induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs) overexpressing either the proangiogenic SDF-1α or anti-inflammatory heme oxygenase-1 (HO-1) in a murine model of MI. ADSCs and hiPSCs were transduced with lentiviral vectors encoding luciferase (Luc), GFP and either HO-1 or SDF-1α. hiPSCs were then differentiated to hiPSC-CMs using small molecules modulating the WNT pathway. Genetically modified ADSCs were firstly administered via intracardiac injection after MI induction in Nude mice. Next, ADSCs-Luc-GFP and genetically modified hiPSC-CMs were injected into the hearts of the more receptive NOD/SCID strain to compare the therapeutic effect of both cell types. Ultrasonography, performed on days 7, 14, 28 and 42, revealed a significant decrease of left ventricular ejection fraction (LVEF) in all MI-induced groups. No improvement of LVEF was observed in ADSC-treated Nude and NOD/SCID mice. In contrast, administration of hiPSC-CMs resulted in a substantial increase of LVEF, occurring between 28 and 42 days after MI, and decreased fibrosis, regardless of genetic modification. Importantly, bioluminescence analysis, as well as immunofluorescent staining, confirmed the presence of hiPSC-CMs in murine tissue. Interestingly, the luminescence signal was strongest in hearts treated with hiPSC-CMs overexpressing HO-1. Performed experiments demonstrate that hiPSC-CMs, unlike ADSCs, are effective in improving heart function after MI. Additionally, long-term evaluation of heart function seems to be crucial for proper assessment of the effect of cell administration.
Keywords: adipose-derived stromal cells; heme oxygenase-1; human induced pluripotent stem cells; myocardial infarction; stromal cell-derived factor-1α.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources

