Expression of Somatostatin Receptor Subtypes (SSTR-1-SSTR-5) in Pediatric Hematological and Oncological Disorders
- PMID: 33297556
- PMCID: PMC7730851
- DOI: 10.3390/molecules25235775
Expression of Somatostatin Receptor Subtypes (SSTR-1-SSTR-5) in Pediatric Hematological and Oncological Disorders
Abstract
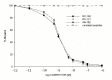
Hematological and oncological disorders represent leading causes of childhood mortality. Neuropeptide somatostatin (SST) has been previously demonstrated in various pediatric tumors, but limited information exists on the expression and characteristics of SST receptors (SSTR) in hematological and oncological disorders of children. We aimed to investigate the expression of mRNA for SSTR subtypes (SSTR-1-5) in 15 pediatric hematological/oncological specimens by RT-PCR. The presence and binding characteristics of SSTRs were further studies by ligand competition assay. Our results show that the pediatric tumor samples highly expressed mRNA for the five SSTR subtypes with various patterns. The mRNA for SSTR-2 was detected in all specimens independently of their histological type. A Hodgkin lymphoma sample co-expressed mRNA for all five SSTR subtypes. SSTR-3 and SSTR-5 were detected only in malignant specimens, such as rhabdomyosarcoma, Hodgkin lymphoma, acute lymphoblastic leukemia, and a single nonmalignant condition, hereditary spherocytosis. The incidence of SSTR-1 and SSTR-4 was similar (60%) in the 15 specimens investigated. Radioligand binding studies demonstrated the presence of specific SSTRs and high affinity binding of SST analogs in pediatric solid tumors investigated. The high incidence of SSTRs in hematological and oncological disorders in children supports the merit of further investigation of SSTRs as molecular targets for diagnosis and therapy.
Keywords: hematological-oncological disorders in children; somatostatin analogs; somatostatin receptors.
Conflict of interest statement
The authors declare no conflict of interests in this work. The founding sponsors had no role in the design of the study, in the collection, analyses, or interpretation of data, in the writing of the manuscript, and in the decision to publish the results.
Figures



References
-
- Drastikova M., Beranek M., Gabalec F., Netuka D., Masopust V., Cesak T., Marek J., Palicka V., Cap J. Expression profiles of somatostatin, dopamine, and estrogen receptors in pituitary adenomas determined by means of synthetic multilocus calibrators. Biomed. Pap. 2016;160:238–243. doi: 10.5507/bp.2015.058. - DOI - PubMed
-
- Schally A.V., Halmos G. Drug Delivery in Oncology. Wiley-VCH Verlag GmbH & Co. KGaA; Weinheim, Germany: 2012. Targeting to peptide receptors; pp. 1219–1261.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

