Clinical trial protocol to evaluate the efficacy of cefixime in the treatment of early syphilis
- PMID: 33298143
- PMCID: PMC7725115
- DOI: 10.1186/s13063-020-04885-z
Clinical trial protocol to evaluate the efficacy of cefixime in the treatment of early syphilis
Abstract
Background: Syphilis rates have been increasing both in the USA and internationally with incidence higher among men-who-have-sex-with-men and people living with human immunodeficiency virus (HIV) infection. Currently, benzathine penicillin is the recommended treatment for syphilis in all patients. Global shortages and cost increases in benzathine penicillin call for alternative treatment options. This study evaluates the efficacy of oral cefixime for the treatment of early syphilis.
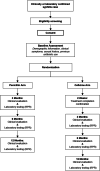
Methods: We are conducting a randomized, multisite, open-label, non-comparative clinical trial in Los Angeles and Oakland, CA. Eligible participants are ≥ 18 years old, with primary, secondary, or early latent syphilis (rapid plasma reagin [RPR] titer ≥ 1:8). Patients with HIV infection must have a viral load ≤ 200 copies/mL and CD4+ T cell count ≥ 350 cells/μL during the past 6 months. Participants are randomized to receive either 2.4 M IU benzathine penicillin G intramuscularly once or cefixime 400 mg orally twice a day for 10 days. Participants return at 3, 6, and 12 months post-treatment for follow-up RPR serological testing. The primary outcome is the proportion of participants who achieve ≥ 4-fold RPR titer decrease at 3 or 6 months post-treatment.
Discussion: Clinical trials evaluating the efficacy of alternative antibiotics to penicillin are urgently needed.
Trial registration: Clinicaltrials.gov NCT03660488 . Registered on 4 September 2018.
Keywords: Cefixime; Clinical trial; Early syphilis; Penicillin; Syphilis; Treponema pallidum.
Figures
References
-
- ClinicalTrials.gov . Cefixime for alternative syphilis treatment. Bethesda: National Library of Medicine (US); 2018.
-
- Braxton J, Grey J, Harvey A, Kidd S, Presley RJ, Ramirez V, et al. Syphilis surveillance supplement 2013–2017 for sexually transmitted disease surveillance 2017. 2019.
-
- World Health Organization . WHO guidelines for the treatment of Treponema pallidum (syphilis) 2016. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous