Treatment of allergic rhinitis during and outside the pollen season using mobile technology. A MASK study
- PMID: 33298191
- PMCID: PMC7726888
- DOI: 10.1186/s13601-020-00342-x
Treatment of allergic rhinitis during and outside the pollen season using mobile technology. A MASK study
Erratum in
-
Corrigendum: Treatment of allergic rhinitis during and outside the pollen season using mobile technology. A MASK study.Clin Transl Allergy. 2022 Mar;12(3):e12126. doi: 10.1002/clt2.12126. Clin Transl Allergy. 2022. PMID: 35344306 Free PMC article. No abstract available.
Abstract
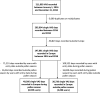
Background: The analysis of mobile health (mHealth) data has generated innovative insights into improving allergic rhinitis control, but additive information is needed. A cross-sectional real-world observational study was undertaken in 17 European countries during and outside the estimated pollen season. The aim was to collect novel information including the phenotypic characteristics of the users.
Methods: The Allergy Diary-MASK-air-mobile phone app, freely available via Google Play and App, was used to collect the data of daily visual analogue scales (VASs) for overall allergic symptoms and medication use. Fluticasone Furoate (FF), Mometasone Furoate (MF), Azelastine Fluticasone Proprionate combination (MPAzeFlu) and eight oral H1-antihistamines were studied. Phenotypic characteristics were recorded at entry. The ARIA severity score was derived from entry data. This was an a priori planned analysis.
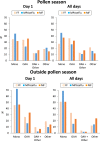
Results: 9037 users filled in 70,286 days of VAS in 2016, 2017 and 2018. The ARIA severity score was lower outside than during the pollen season. Severity was similar for all treatment groups during the pollen season, and lower in the MPAzeFlu group outside the pollen season. Days with MPAzeFlu had lower VAS levels and a higher frequency of monotherapy than the other treatments during the season. Outside the season, days with MPAzeFlu also had a higher frequency of monotherapy. The number of reported days was significantly higher with MPAzeFlu during and outside the season than with MF, FF or oral H1-antihistamines.
Conclusions: This study shows that the overall efficacy of treatments is similar during and outside the pollen season and indicates that medications are similarly effective during the year.
Keywords: Allergic rhinitis; Anti-histamines; Corticosteroids; ICT; MASK; Mobile health; Treatment.
Conflict of interest statement
Dr. Almeida reports grants from ERDF (European Regional Development Fund) through the operation POCI-01-0145-FEDER-029130 (“mINSPIRERS—mHealth para medição e melhoria da adesão à medicação nas doenças respiratórias obstrutivas crónicas—generalização e avaliação de tecnologias de gamificação, suporte por pares e processamento avançado de imagem”) funded by the Programa Operacional Competitividade e Internacionalização—COMPETE2020 and by National Funds through FCT (Fundação para a Ciência e a Tecnologia, during the conduct of the study.Dr. Ansotegui reports personal fees from Hikma, Roxall, Astra Zeneca, Menarini, UCB, Faes Farma, Sanofi, Mundipharma, outside the submitted work.Bachert: Meda, Stallergenes and ALK (speaker). J Bousquet reports personal fees and other from Chiesi, Cipla, Hikma, Menarini, Mundipharma, Mylan, Novartis, Sanofi-Aventis, Takeda, Teva, Uriach, outside the submitted work. other from Kyomed. Dr. Bosnic-Anticevich reports personal fees from Teva, Boehringer Ingelheim, Sanofi, GSK, AstraZeneca, outside the submitted work. Dr. Cardona reports personal fees from ALK, Allergopharma, Allergy Therapeutics, Diater, LETI, Thermofisher, Stallergenes, outside the submitted work. Dr. Cecchi reports personal fees from Menarini, Malesci, ALK Abellò, Menarini, non-financial support from Mundipharma, outside the submitted work.Dr. Correia-de-Sousa reports other from Boheringer Ingelheim, GSK, AstraZeneca, outside the submitted work. Dr. Cruz reports grants and personal fees from Astrazeneca, grants from GSK, personal fees from Boehringer Ingelheim, CHIESI, NOVARTIS, Eurofarma, MEDA Pharma, Boston Scientific, outside the submitted work. Dr. Devillier reports personal fees and non-financial support from Astra Zeneca, Mylan, Stallergenes Greer, personal fees from ALK Abello, Menarini, GlaxoSmithKline, Sanofi, outside the submitted work. Dr. Haahtela reports personal fees from Mundipharma, Novartis, and Orion Pharma, outside the submitted work. Dr. Eller reports other from Thermofisher Scientific, outside the submitted work. Dr. Fonseca reports personal fees from AstraZeneca, GSK, Novartis, Teva, grants from Mundipharma, outside the submitted work. Dr. Ivancevich reports personal fees from Faes Farma Euro-Farma Argentina, other from Lboratorios Casasco, Sanofi, outside the submitted work. Dr. Fokkens reports grants from Mylan, ALK, Allergy Therapeutics, GSK, Sanofi, Novartis, outside the submitted work. Dr. Klimek reports personal fees from MEDA, Sweden, Boehringer Ingelheim, Germany, grants and personal fees from ALK Abelló, Denmark, Novartis, Switzerland, Allergopharma, Germany, Bionorica, Germany, GSK, Great Britain, Lofarma, Italy, grants from Biomay, Austria, HAL, Netherlands, LETI, Spain, Roxall, Germany Bencard, Great Britain, outside the submitted work. Dr. Kuna reports personal fees from Adamed, Boehringer Ingelheim, AstraZeneca, Chiesi, FAES, Berlin Chemie, Novartis, Polpharma, Allergopharma,outside the submitted work. Dr Kvedariene has received payment for consultancy from GSK and for lectures from StallergensGreer, Berlin-CHemie outside the submitted work. Dr. Larenas Linnemann reports personal fees from Amstrong, Astrazeneca, Boehringer Ingelheim, Chiesi, DBV Technologies, Grunenthal, GSK, MEDA, Menarini, MSD, Novartis, Pfizer, Sanofi, Siegfried, UCB, grants from Sanofi, Astrazeneca, Novartis, UCB, GSK, TEVA, Boehringer Ingelheim, Chiesi, outside the submitted work. Dr. Melén reports personal fees from Advisory board reimbursement from Novartis, outside the submitted work. Dr. Ralph Mösges reports personal fees from ALK, allergopharma, Allergy Therapeutics, Hexal, personal fees from Servier, personal fees from Klosterfrau, Stada, UCB, Friulchem, grants from ASIT biotech, Nuvo, Bayer, FAES, GSK, MSD, Johnson&Johnson, Meda, Optima, Ursapharm, BitopAG, Hulka, grants and personal fees from Bencard, grants from Leti, Stallergenes, grants, personal fees and non-financial support from Lofarma, non-financial support from Roxall, from Atmos, from Bionorica, Otonomy, Ferrero, personal fees and non-financial support from Novartis, outside the submitted work. Dr. Papadopoulos reports grants from Gerolymatos, personal fees from Hal Allergy B.V., Novartis Pharma AG, Menarini, Hal Allergy B.V., Mylan, outside the submitted work. Dr. Pfaar reports grants and personal fees from ALK-Abelló, Allergopharma, Stallergenes Greer, HAL Allergy Holding B.V./HAL Allergie GmbH, Bencard Allergie GmbH/Allergy Therapeutics, Lofarma, ASIT Biotech Tools S.A., Laboratorios LETI/LETI Pharma, Anergis S.A., grants from Glaxo Smith Kline, Biomay, Nuvo, Circassia, personal fees from MEDA Pharma/MYLAN, Mobile Chamber Experts (a GA2LEN Partner Indoor Biotechnologies, Astellas Pharma Global, outside the submitted work. Dr. Ryan reports personal fees from Mylan, AZ, AZ, GSK, from Chiesi, BI, grants from Mylan, outside the submitted work.Dr Schmid-Grendelmeier reports honarium and consultancy fees from ALK-Abello, Allergopharma, Bencard, MEDA and Stallergenes Dr. Stelmach reports grants from São Paulo Research Foundation, MSD, grants and personal fees from Novartis, grants, personal fees and non-financial support from AstraZeneca, Chiesi, personal fees and non-financial support from Boheringer Ingelheim, outside the submitted work. Dr. Todo-Bom reports grants and personal fees from Novartis, Mundipharma, GSK Teva Pharma, personal fees from AstraZeneca, grants from Boehringer Ingelheim, Sanofi, Leti, outside the submitted work. Dr. Toppila-Salmi reports other from Biomedical Systems Ltd., Roche Ltd., grants from Erkko Foundation, outside the submitted work. Dr. Waserman reports other from CSL Behring Shire, AstraZeneca, Teva, Meda, other from Merck, GSK, Novartis, Pediapharm, Aralez Sanofi, Stallergenes, outside the submitted work.
Figures
References
-
- Bedard A, Basagana X, Anto JM, Garcia-Aymerich J, Devillier P, Arnavielhe S, et al. Mobile technology offers novel insights into the control and treatment of allergic rhinitis: The MASK study. J Allergy Clin Immunol. 2019;144(1):135-43 e6. - PubMed
Publication types
LinkOut - more resources
Full Text Sources