Amylases in the Human Vagina
- PMID: 33298571
- PMCID: PMC7729256
- DOI: 10.1128/mSphere.00943-20
Amylases in the Human Vagina
Abstract
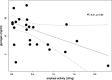
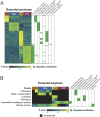
Dominance of Lactobacillus species in vaginal communities is a hallmark of healthy conditions in the female genital tract. Key nutrients for lactobacilli include sugars produced when glycogen is degraded by α-amylase in the vagina. While α-amylase activity has been demonstrated in vaginal fluids, it is unclear whether α-amylases are produced solely by the host, bacteria in the vagina, or both. We screened cervicovaginal mucus from 23 reproductive-age women, characterized the species composition of vaginal communities, measured vaginal pH, and determined levels of amylase activity, glycogen, and lactic acid. Based on differences in these measured variables, one sample from each of four individual donors was selected for metagenomic and proteomic analyses. Of eight putative bacterial amylases identified in the assembled bacterial metagenomes, we detected four in vaginal fluids. These amylases were produced by various bacteria in different vaginal communities. Moreover, no two communities were the same in terms of which bacteria were producing amylases. Although we detected bacterial amylases in vaginal fluids, there was no clear association between the bacterial species that was dominant in a community and the level of amylase activity. This association was likely masked by the presence of human α-amylase, which was also detected in vaginal fluids. Finally, the levels of amylase activity and glycogen were only weakly associated. Our findings show, for the first time, that multiple amylases from both bacterial and human origins can be present simultaneously in the vagina. This work also suggests that the link between glycogen, amylase, and Lactobacillus in the vagina is complex.IMPORTANCE In this study, we show that multiple bacteria in the vaginal community produce amylases that hydrolyze glycogen into simpler sugars (i.e., maltose and maltotriose). These sugars serve as "common goods" that sustain bacterial populations in vaginal communities. Given the temporal changes that are observed in the human vaginal microbiome, we expect the kinds of bacterial amylases produced will also vary over time. These differences influence the pool of resources that are broadly shared and shape the species composition of the vaginal bacterial community.
Keywords: Lactobacillus; amylase; glycogen; vaginal microbiome.
Copyright © 2020 Nunn et al.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
