BCL-XL is an actionable target for treatment of malignant pleural mesothelioma
- PMID: 33298868
- PMCID: PMC7603509
- DOI: 10.1038/s41420-020-00348-1
BCL-XL is an actionable target for treatment of malignant pleural mesothelioma
Erratum in
-
Correction: BCL-XL is an actionable target for treatment of malignant pleural mesothelioma.Cell Death Discov. 2021 Jun 15;7(1):146. doi: 10.1038/s41420-020-00388-7. Cell Death Discov. 2021. PMID: 34131107 Free PMC article. No abstract available.
Abstract
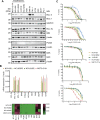
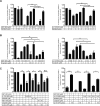
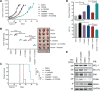
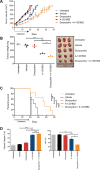
Despite having one of the lowest survival rates of all cancers, there have been no new approved treatments for malignant pleural mesothelioma (MPM) in over a decade. Standard-of-care treatment relies on Cisplatin plus Pemetrexed chemotherapy. Here, we tested a suite of BH3-mimetic drugs targeting BCL-2 pro-survival proteins of the intrinsic apoptotic pathway. We found BCL-XL is the dominant pro-survival protein in a panel of cell lines in vitro, though potent, synergistic cell killing occurred with MCL-1 co-targeting. This correlates with high-level expression of BCL-XL and MCL-1 in cell lines and a large cohort of patient tumour samples. BCL-XL inhibition combined with Cisplatin also enhanced cell killing. In vivo BCL-XL inhibition was as effective as Cisplatin, and the combination enhanced tumour growth control and survival. Genetic ablation of MCL-1 also enhanced the effects of BCL-XL inhibitors, in vivo. Combined, these data provide a compelling rationale for the clinical investigation of BH3-mimetics targeting BCL-XL in MPM.
Conflict of interest statement
W.D.F. and E.F.L. were previously employees of The Walter and Eliza Hall Institute where they were involved in collaborations with AbbVie and Genentech to develop and characterise BH3-mimetic drugs and receive payments in respect of Venetoclax. They have an on-going collaboration with Astra Zeneca studying BH3-mimetics. S.A. has received speaker fees and travel support from Merck-Sharpe Dohme, Astra Zeneca, Boehringer-Ingelheim, Bristol-Myers Squibb and Roche, outside the submitted work. J.C. has received speaker fees and travel support from Amgen, Bristol-Myers Squibb, GlaxoSmith Kline, Merck; Merck Sharp & Dohme and Novartis, outside the submitted work. T.J. has received speaker fees and travel support from Pfizer, Astra Zeneca, BMS, Novartis, Merck Sharp & Dohme, Boehringer-Ingelheim, Takeda, Merck and Roche, outside the submitted work.
Figures


References
-
- Abdel-Rahman, O. Global trends in mortality from malignant mesothelioma: analysis of WHO mortality database (1994–2013). Clin. Respir. J.12, 2090–2100 (2018). - PubMed
-
- Tsao AS, et al. Current and future management of malignant mesothelioma: a consensus report from the National Cancer Institute Thoracic Malignancy Steering Committee, International Association for the Study of Lung Cancer, and Mesothelioma Applied Research Foundation. J. Thorac. Oncol. 2018;13:1655–1667. doi: 10.1016/j.jtho.2018.08.2036. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

