Nature-inspired remodeling of (aza)indoles to meta-aminoaryl nicotinates for late-stage conjugation of vitamin B3 to (hetero)arylamines
- PMID: 33298909
- PMCID: PMC7726565
- DOI: 10.1038/s41467-020-19610-2
Nature-inspired remodeling of (aza)indoles to meta-aminoaryl nicotinates for late-stage conjugation of vitamin B3 to (hetero)arylamines
Abstract
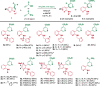
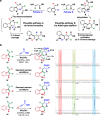
Despite the availability of numerous routes to substituted nicotinates based on the Bohlmann-Rahtz pyridine synthesis, the existing methods have several limitations, such as the inevitable ortho-substitutions and the inability to conjugate vitamin B3 to other pharmaceutical agents. Inspired by the biosynthesis of nicotinic acid (a form of vitamin B3) from tryptophan, we herein report the development of a strategy for the synthesis of meta-aminoaryl nicotinates from 3-formyl(aza)indoles. Our strategy is mechanistically different from the reported routes and involves the transformation of (aza)indole scaffolds into substituted meta-aminobiaryl scaffolds via Aldol-type addition and intramolecular cyclization followed by C-N bond cleavage and re-aromatization. Unlike previous synthetic routes, this biomimetic method utilizes propiolates as enamine precursors and thus allows access to ortho-unsubstituted nicotinates. In addition, the synthetic feasibility toward the halo-/boronic ester-substituted aminobiaryls clearly differentiates the present strategy from other cross-coupling strategies. Most importantly, our method enables the late-stage conjugation of bioactive (hetero)arylamines with nicotinates and nicotinamides and allows access to the previously unexplored chemical space for biomedical research.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

