Condensation of Rubisco into a proto-pyrenoid in higher plant chloroplasts
- PMID: 33298923
- PMCID: PMC7726157
- DOI: 10.1038/s41467-020-20132-0
Condensation of Rubisco into a proto-pyrenoid in higher plant chloroplasts
Abstract
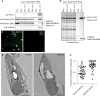
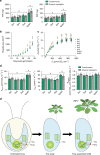
Photosynthetic CO2 fixation in plants is limited by the inefficiency of the CO2-assimilating enzyme Rubisco. In most eukaryotic algae, Rubisco aggregates within a microcompartment known as the pyrenoid, in association with a CO2-concentrating mechanism that improves photosynthetic operating efficiency under conditions of low inorganic carbon. Recent work has shown that the pyrenoid matrix is a phase-separated, liquid-like condensate. In the alga Chlamydomonas reinhardtii, condensation is mediated by two components: Rubisco and the linker protein EPYC1 (Essential Pyrenoid Component 1). Here, we show that expression of mature EPYC1 and a plant-algal hybrid Rubisco leads to spontaneous condensation of Rubisco into a single phase-separated compartment in Arabidopsis chloroplasts, with liquid-like properties similar to a pyrenoid matrix. This work represents a significant initial step towards enhancing photosynthesis in higher plants by introducing an algal CO2-concentrating mechanism, which is predicted to significantly increase the efficiency of photosynthetic CO2 uptake.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

