Predicting breast cancer response to neoadjuvant treatment using multi-feature MRI: results from the I-SPY 2 TRIAL
- PMID: 33298938
- PMCID: PMC7695723
- DOI: 10.1038/s41523-020-00203-7
Predicting breast cancer response to neoadjuvant treatment using multi-feature MRI: results from the I-SPY 2 TRIAL
Abstract
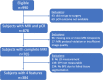
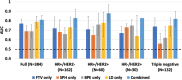
Dynamic contrast-enhanced (DCE) MRI provides both morphological and functional information regarding breast tumor response to neoadjuvant chemotherapy (NAC). The purpose of this retrospective study is to test if prediction models combining multiple MRI features outperform models with single features. Four features were quantitatively calculated in each MRI exam: functional tumor volume, longest diameter, sphericity, and contralateral background parenchymal enhancement. Logistic regression analysis was used to study the relationship between MRI variables and pathologic complete response (pCR). Predictive performance was estimated using the area under the receiver operating characteristic curve (AUC). The full cohort was stratified by hormone receptor (HR) and human epidermal growth factor receptor 2 (HER2) status (positive or negative). A total of 384 patients (median age: 49 y/o) were included. Results showed analysis with combined features achieved higher AUCs than analysis with any feature alone. AUCs estimated for the combined versus highest AUCs among single features were 0.81 (95% confidence interval [CI]: 0.76, 0.86) versus 0.79 (95% CI: 0.73, 0.85) in the full cohort, 0.83 (95% CI: 0.77, 0.92) versus 0.73 (95% CI: 0.61, 0.84) in HR-positive/HER2-negative, 0.88 (95% CI: 0.79, 0.97) versus 0.78 (95% CI: 0.63, 0.89) in HR-positive/HER2-positive, 0.83 (95% CI not available) versus 0.75 (95% CI: 0.46, 0.81) in HR-negative/HER2-positive, and 0.82 (95% CI: 0.74, 0.91) versus 0.75 (95% CI: 0.64, 0.83) in triple negatives. Multi-feature MRI analysis improved pCR prediction over analysis of any individual feature that we examined. Additionally, the improvements in prediction were more notable when analysis was conducted according to cancer subtype.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Lobbes M, Prevos R, Smidt M. Response monitoring of breast cancer patients receiving neoadjuvant chemotherapy using breast MRI – a review of current knowledge. J. Cancer Ther. Res. 2012;1:34. doi: 10.7243/2049-7962-1-34. - DOI
-
- Hylton NM. Vascularity assessment of breast lesions with gadolinium-enhanced MR imaging. Magn. Reson. Imaging Clin. N. Am. 1999;7:411–20. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

