Mucosal delivery of a multistage subunit vaccine promotes development of lung-resident memory T cells and affords interleukin-17-dependent protection against pulmonary tuberculosis
- PMID: 33298977
- PMCID: PMC7665186
- DOI: 10.1038/s41541-020-00255-7
Mucosal delivery of a multistage subunit vaccine promotes development of lung-resident memory T cells and affords interleukin-17-dependent protection against pulmonary tuberculosis
Abstract
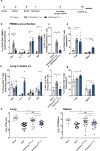
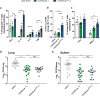
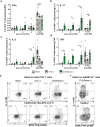
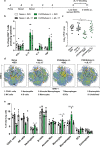
The development of effective vaccines against bacterial lung infections requires the induction of protective, pathogen-specific immune responses without deleterious inflammation within the pulmonary environment. Here, we made use of a polysaccharide-adjuvanted vaccine approach to elicit resident pulmonary T cells to protect against aerosol Mycobacterium tuberculosis infection. Intratracheal administration of the multistage fusion protein CysVac2 and the delta-inulin adjuvant Advax™ (formulated with a TLR9 agonist) provided superior protection against aerosol M. tuberculosis infection in mice, compared to parenteral delivery. Surprisingly, removal of the TLR9 agonist did not impact vaccine protection despite a reduction in cytokine-secreting T cell subsets, particularly CD4+IFN-γ+IL-2+TNF+ multifunctional T cells. CysVac2/Advax-mediated protection was associated with the induction of lung-resident, antigen-specific memory CD4+ T cells that expressed IL-17 and RORγT, the master transcriptional regulator of Th17 differentiation. IL-17 was identified as a key mediator of vaccine efficacy, with blocking of IL-17 during M. tuberculosis challenge reducing phagocyte influx, suppressing priming of pathogen-specific CD4+ T cells in local lymph nodes and ablating vaccine-induced protection. These findings suggest that tuberculosis vaccines such as CysVac2/Advax that are capable of eliciting Th17 lung-resident memory T cells are promising candidates for progression to human trials.
Conflict of interest statement
N.P. is the research director for Vaxine P/L. E.L.S. was an employee of Vaxine P/L. The remaining authors declare that they have no competing interests.
Figures



References
-
- World Health Organisation. Global Tuberculosis Report 2019 (WHO, 2019).
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

