Immunohistochemical detection of PAX-FOXO1 fusion proteins in alveolar rhabdomyosarcoma using breakpoint specific monoclonal antibodies
- PMID: 33299109
- PMCID: PMC9253961
- DOI: 10.1038/s41379-020-00719-0
Immunohistochemical detection of PAX-FOXO1 fusion proteins in alveolar rhabdomyosarcoma using breakpoint specific monoclonal antibodies
Abstract
Alveolar Rhabdomyosarcoma (ARMS) is an aggressive pediatric cancer with about 80% of cases characterized by either a t(1;13)(p36;q14) or t(2;13)(q35;q14), which results in the formation of the fusion oncogenes PAX7-FOXO1 and PAX3-FOXO1, respectively. Since patients with fusion-positive ARMS (FP-RMS) have a poor prognosis and are treated with an aggressive therapeutic regimen, correct classification is of clinical importance. Detection of the translocation by different molecular methods is used for diagnostics, including fluorescence in situ hybridization and RT-PCR or NGS based approaches. Since these methods are complex and time consuming, we developed specific monoclonal antibodies (mAbs) directed to the junction region on the PAX3-FOXO1 fusion protein. Two mAbs, PFM.1 and PFM.2, were developed and able to immunoprecipitate in vitro-translated PAX3-FOXO1 and cellular PAX3-FOXO1 from FP-RMS cells. Furthermore, the mAbs recognized a 105 kDa band in PAX3-FOXO1-transfected cells and in FP-RMS cell lines. The mAbs did not recognize proteins in fusion-negative embryonal rhabdomyosarcoma cell lines, nor did they recognize PAX3 or FOXO1 alone when compared to anti-PAX3 and anti-FOXO1 antibodies. We next evaluated the ability of mAb PFM.2 to detect the fusion protein by immunohistochemistry. Both PAX3-FOXO1 and PAX7-FOXO1 were detected in HEK293 cells transfected with the corresponding cDNAs. Subsequently, we stained 26 primary tumor sections and a rhabdomyosarcoma tissue array and detected both fusion proteins with a positive predictive value of 100%, negative predictive value of 98%, specificity of 100% and a sensitivity of 91%. While tumors are stained homogenously in PAX3-FOXO1 cases, the staining pattern is heterogenous with scattered positive cells only in tumors expressing PAX7-FOXO1. No staining was observed in stromal cells, embryonal rhabdomyosarcoma, and fusion-negative rhabdomyosarcoma. These results demonstrate that mAbs specific for the chimeric oncoproteins PAX3-FOXO1 and PAX7-FOXO1 can be used efficiently for simple and fast subclassification of rhabdomyosarcoma in routine diagnostics via immunohistochemical detection.
Conflict of interest statement
Figures

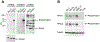




References
-
- Pappo AS. Rhabdomyosarcoma and other soft tissue sarcomas in children. Curr Opin Oncol. 1996;8:311–6. - PubMed
-
- WHO Classification of Tumours Editorial Board. Soft tissue and bone tumours: WHO classification of tumours. 5th ed. Lyon: IARC Press; 2020.
-
- Barr FG, Galili N, Holick J, Biegel JA, Rovera G, Emanuel BS. Rearrangement of the PAX3 paired box gene in the paediatric solid tumour alveolar rhabdomyosarcoma. Nat Genet. 1993;3:113–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

