Let Sleeping Patients Lie, avoiding unnecessary overnight vitals monitoring using a clinically based deep-learning model
- PMID: 33299116
- PMCID: PMC7666176
- DOI: 10.1038/s41746-020-00355-7
Let Sleeping Patients Lie, avoiding unnecessary overnight vitals monitoring using a clinically based deep-learning model
Abstract
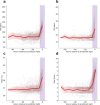
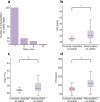
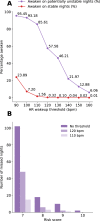
Impaired sleep for hospital patients is an all too common reality. Sleep disruptions due to unnecessary overnight vital sign monitoring are associated with delirium, cognitive impairment, weakened immunity, hypertension, increased stress, and mortality. It is also one of the most common complaints of hospital patients while imposing additional burdens on healthcare providers. Previous efforts to forgo overnight vital sign measurements and improve patient sleep used providers' subjective stability assessment or utilized an expanded, thus harder to retrieve, set of vitals and laboratory results to predict overnight clinical risk. Here, we present a model that incorporates past values of a small set of vital signs and predicts overnight stability for any given patient-night. Using data obtained from a multi-hospital health system between 2012 and 2019, a recurrent deep neural network was trained and evaluated using ~2.3 million admissions and 26 million vital sign assessments. The algorithm is agnostic to patient location, condition, and demographics, and relies only on sequences of five vital sign measurements, a calculated Modified Early Warning Score, and patient age. We achieved an area under the receiver operating characteristic curve of 0.966 (95% confidence interval [CI] 0.956-0.967) on the retrospective testing set, and 0.971 (95% CI 0.965-0.974) on the prospective set to predict overnight patient stability. The model enables safe avoidance of overnight monitoring for ~50% of patient-nights, while only misclassifying 2 out of 10,000 patient-nights as stable. Our approach is straightforward to deploy, only requires regularly obtained vital signs, and delivers easily actionable clinical predictions for a peaceful sleep in hospitals.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

