Neddylation of PTEN regulates its nuclear import and promotes tumor development
- PMID: 33299139
- PMCID: PMC8027835
- DOI: 10.1038/s41422-020-00443-z
Neddylation of PTEN regulates its nuclear import and promotes tumor development
Erratum in
-
Author Correction: Neddylation of PTEN regulates its nuclear import and promotes tumor development.Cell Res. 2021 Mar;31(3):374. doi: 10.1038/s41422-021-00470-4. Cell Res. 2021. PMID: 33514915 Free PMC article. No abstract available.
Abstract
PTEN tumor suppressor opposes the PI3K/Akt signaling pathway in the cytoplasm and maintains chromosomal integrity in the nucleus. Nucleus-cytoplasm shuttling of PTEN is regulated by ubiquitylation, SUMOylation and phosphorylation, and nuclear PTEN has been proposed to exhibit tumor-suppressive functions. Here we show that PTEN is conjugated by Nedd8 under high glucose conditions, which induces PTEN nuclear import without effects on PTEN stability. PTEN neddylation is promoted by the XIAP ligase and removed by the NEDP1 deneddylase. We identify Lys197 and Lys402 as major neddylation sites on PTEN. Neddylated PTEN accumulates predominantly in the nucleus and promotes rather than suppresses cell proliferation and metabolism. The nuclear neddylated PTEN dephosphorylates the fatty acid synthase (FASN) protein, inhibits the TRIM21-mediated ubiquitylation and degradation of FASN, and then promotes de novo fatty acid synthesis. In human breast cancer tissues, neddylated PTEN correlates with tumor progression and poor prognosis. Therefore, we demonstrate a previously unidentified pool of nuclear PTEN in the Nedd8-conjugated form and an unexpected tumor-promoting role of neddylated PTEN.
Conflict of interest statement
The authors declare no competing interests.
Figures

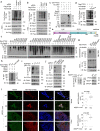
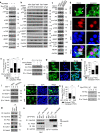
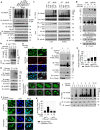



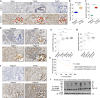

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

