Arterialization requires the timely suppression of cell growth
- PMID: 33299176
- PMCID: PMC7116692
- DOI: 10.1038/s41586-020-3018-x
Arterialization requires the timely suppression of cell growth
Abstract
The formation of arteries is thought to occur by the induction of a highly conserved arterial genetic programme in a subset of vessels that will later experience an increase in oxygenated blood flow1,2. The initial steps of arterial specification require both the VEGF and Notch signalling pathways3-5. Here, we combine inducible genetic mosaics and transcriptomics to modulate and define the function of these signalling pathways in cell proliferation, arteriovenous differentiation and mobilization. We show that endothelial cells with high levels of VEGF or Notch signalling are intrinsically biased to mobilize and form arteries; however, they are not genetically pre-determined, and can also form veins. Mechanistically, we found that increased levels of VEGF and Notch signalling in pre-arterial capillaries suppresses MYC-dependent metabolic and cell-cycle activities, and promotes the incorporation of endothelial cells into arteries. Mosaic lineage-tracing studies showed that endothelial cells that lack the Notch-RBPJ transcriptional activator complex rarely form arteries; however, these cells regained the ability to form arteries when the function of MYC was suppressed. Thus, the development of arteries does not require the direct induction of a Notch-dependent arterial differentiation programme, but instead depends on the timely suppression of endothelial cell-cycle progression and metabolism, a process that precedes arterial mobilization and complete differentiation.
Conflict of interest statement
Figures
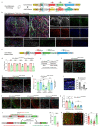

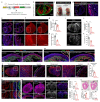


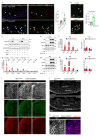
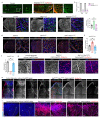

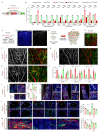




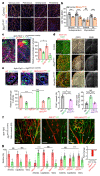
Comment in
-
Stop the Divide and Build Coronary Arteries.Dev Cell. 2021 Feb 8;56(3):255-256. doi: 10.1016/j.devcel.2021.01.008. Dev Cell. 2021. PMID: 33561420 Free PMC article.
References
-
- Phng LK, Gerhardt H. Angiogenesis: a team effort coordinated by notch. Dev Cell. 2009;16:196–208. - PubMed
-
- Benedito R, Hellström M. Notch as a hub for signaling in angiogenesis. Exp Cell Res. 2013;319:1281–1288. - PubMed
-
- Lawson ND, Vogel AM, Weinstein BM. Sonic hedgehog and vascular endothelial growth factor act upstream of the Notch pathway during arterial endothelial differentiation. Dev Cell. 2002;3:127–136. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

