Review of Adjuvant Therapies in Renal Cell Carcinoma: Evidence to Date
- PMID: 33299326
- PMCID: PMC7721274
- DOI: 10.2147/OTT.S174149
Review of Adjuvant Therapies in Renal Cell Carcinoma: Evidence to Date
Abstract
In 2018, there were 400,000 new cases of renal cell carcinoma (RCC) globally, with 175,000 deaths attributable to the disease. Three quarters of patients have potentially curable localised disease at diagnosis; however, recurrence rates are as high as 40% following surgery. There are currently no adjuvant therapies in routine clinical use which reliably improve outcomes. Effective adjuvant therapy is an urgent unmet need to reduce recurrence risk and improve outcomes. Early efforts explored chemotherapy, radiotherapy, cytokine therapy, hormonal treatments and tumour cell vaccines as adjuvant therapies, however, have yielded disappointing results. More recently, interest shifted to evaluating tyrosine kinase inhibitors (TKIs) in the adjuvant setting, as they improve outcomes in metastatic disease. Five phase III clinical trials testing adjuvant use of a range of TKIs have been performed, with the results of a sixth trial awaited. Unfortunately, these studies have thus far yielded conflicting and disappointing results, and there is currently no strong evidence for routine adjuvant TKI therapy. In parallel, novel immunotherapy treatment approaches have recently been developed, transforming the management of a range of malignancies, particularly through immune checkpoint inhibitors (ICIs). These approaches are well established in the metastatic context in RCC, as well as in the adjuvant treatment of melanoma. On this basis, five phase III trials are currently ongoing to test the efficacy of a range of ICIs in adjuvant RCC patients, with initial results expected over the next few years. In this article, we review the current evidence for adjuvant therapies in RCC, discuss ongoing clinical trials and suggest directions for future work to address this unmet need.
Keywords: adjuvant therapy; immune checkpoint inhibitors; renal cell carcinoma; tyrosine kinase inhibitors.
© 2020 Tacconi et al.
Conflict of interest statement
The authors report no conflicts of interest for this work.
Figures
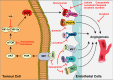
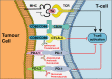
References
-
- CRUK. Kidney cancer statistics. Available from: https://www.cancerresearchuk.org/health-professional/cancer-statistics/s.... Accessed March, 2020.
-
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. - PubMed
-
- Dizman N, Adashek JJ, Hsu J, Bergerot PG, Bergerot CD, Pal SK. Adjuvant treatment in renal cell carcinoma. Clin Adv Hematol Oncol. 2018;16(8):555–563. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

