A Combined Effect of Expression Levels of Obesity-Related Genes and Clinical Factors on Cancer Survival Rate
- PMID: 33299884
- PMCID: PMC7707943
- DOI: 10.1155/2020/8838676
A Combined Effect of Expression Levels of Obesity-Related Genes and Clinical Factors on Cancer Survival Rate
Abstract
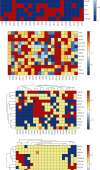
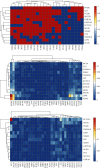
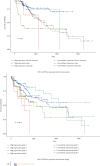
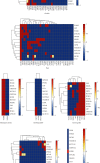
Obesity is directly associated with the risk of cancer in different organs, including breast, colon, and kidney. However, adipocytes could be utilized to control progression for some types of cancer, such as leukemia and breast cancer. To explore the potential correlation between adipocytes and cancer, the combined effect of expression levels of obesity-related genes and clinical factors (i.e., gender, race, menopausal status, history of smoking, tumor grade, body mass index (BMI), and history of drinking) on cancer survival rate was systemically studied. The expression levels of obesity-related genes in cancer tissues and normal tissues were downloaded from The Cancer Genome Atlas (TCGA). Kaplan-Meier curves were plotted using R programming language. The log-rank test was applied to explore the correlation between different clinical subgroups. The overexpression of the nine obesity-related genes (MC4R, TMEM18, KCTD15, GNPDA2, SH2B1, MTCH2, FTO, PCSK1, and GPR120) may associate with tumor-promoting factors in some organs (head and neck, gastrointestinal tract, liver, and gallbladder). Underexpressed LEPR, NEGR1, TMEM18, and SH2B1 genes prevented the progression and metastasis of kidney cancer. The combined effect of clinical factors and the expression levels of obesity-related genes on patients' survival was found to be significant. Our outcomes suggested that the alternations of DNA methylation patterns could result in the changes of expression levels of obesity-related genes, playing a critical role in tumor progression. The results of the current study may be utilized to supplement precision and personalized medicine, as well as provide novel insights for the development of treatment approaches for cancer.
Copyright © 2020 Ting Huang et al.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Figures


References
-
- Rahib L., Smith B. D., Aizenberg R., Rosenzweig A. B., Fleshman J. M., Matrisian L. M. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Research. 2014;74(11):2913–2921. doi: 10.1158/0008-5472.CAN-14-0155. - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

